Biofuel made from woody waste such as sawdust can serve as a carbon-neutral alternative to petroleum-based fuels. But the economics of producing this type of biofuel don’t work without policy measures such as carbon taxes or other subsidies, which can be politically difficult to enact. Now researchers have found a way to greatly increase the […]
Biofuel made from woody waste such as sawdust can serve as a carbon-neutral alternative to petroleum-based fuels. But the economics of producing this type of biofuel don’t work without policy measures such as carbon taxes or other subsidies, which can be politically difficult to enact. Now researchers have found a way to greatly increase the value of one type of biofuel production from woody waste: by converting a by-product of the process into highly pure graphite that’s good enough quality to use in lithium-ion battery electrodes.
Pyrolysis, which uses high temperatures in the absence of oxygen to break down organic materials, is one of the most efficient ways to convert woody plant waste into heating and transportation fuels, says Michael J. Wagner, a nanochemist at George Washington University. The process breaks down the sturdy, lignocellulose polymer that makes wood and other tough plants strong. Pyrolysis produces both oil that can be refined into diesel fuel, and a charcoal-like by-product called biochar.
Although pyrolysis is the cheapest process for turning lignocellulosic biomass into bio-oil, it’s still not economical, Wagner says. So he and other researchers have been exploring ways to add value to the process by finding uses for biochar. While trying to find useful things to do with biochar, Wagner and his colleagues found they could make graphite.
They started by making the biochar. They mixed hardwood sawdust with iron microparticles and pressed them into 20-mm-diameter pellets. After drilling a hole in the center of the pellets, the researchers heated them to 600 °C in a nitrogen atmosphere. This pyrolysis left behind biochar nuggets that were about 40% of the mass of the original pellets.
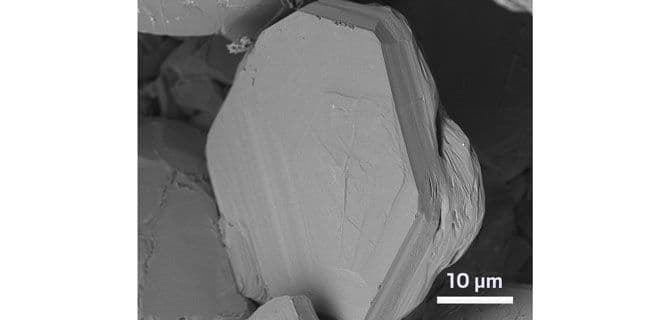
Next, the researchers used a tool they knew could help transform the organic material into other kinds of carbon-based molecules: an infrared laser. Using the predrilled holes, they put the biochar pellets on metal skewers and rotated them while zapping them with the laser—analogous to the way gyro meat on a skewer rotates under a heating element. When the researchers shaved off the top layer of the pellet and examined it, they found they had made graphite.
At first, Wagner wasn’t too excited—graphite is a pretty quotidian material. “Then I did a back of the envelope calculation,” he says. “Wait, it’s cheap! It’s good quality!” The biochar graphite was what battery chemists call “potato flake” graphite, made up of stacked, aligned sheets of graphene in which each carbon atom nests in an empty spot in the layers above and below. Making high-purity graphite, with just the right structure needed for battery electrodes, is not possible with most organic feedstocks. Impurities in the starting material or extra cross-linking during synthesis can prevent the graphene sheets from sliding into place to make those perfect potato flakes. Wagner says he suspects the laser pulses help the graphene sheets align during synthesis.
The researchers washed the pellets in acid to dissolve the iron, processed the graphite to make electrodes, and tested the electrodes in coin-sized lithium-ion batteries. When they milled the graphite to pack the particles more densely, the biochar-derived electrodes performed as well as those made from conventionally sourced graphite, which is typically mined or made from certain forms of coal. According to his “crude economic model,” Wagner projects that making battery-quality graphite would raise the value of the process’s products by three orders of magnitude.
“This may indeed change the economics of pyrolysis as an energy pathway,” says Johannes Lehmann, a biogeochemist at Cornell University who researches ways to use biochar to mitigate climate change. Turning low-value biochar byproduct into high-value materials such as battery-quality graphite could make pyrolysis more realistic, he says.
Wagner says the next step is to scale up the process to produce kilograms of graphite, which should be possible because he says all the required techniques are currently used in various industrial processes.
This article is reproduced with permission from C&EN (© American Chemical Society). The article was first published on August 29, 2018.
