How can scientists bridge scientific knowledge and next-generation risk evaluations without experimenting on animals? According to Professor Ans Punt of RIKILT Wageningen University and Research in the Netherlands, the answer lies in promoting the collective experience with quantitative kinetics. “Quantitative predictions of in vivo chemical levels based on in vitro data will become a cornerstone […]

How can scientists bridge scientific knowledge and next-generation risk evaluations without experimenting on animals? According to Professor Ans Punt of RIKILT Wageningen University and Research in the Netherlands, the answer lies in promoting the collective experience with quantitative kinetics.
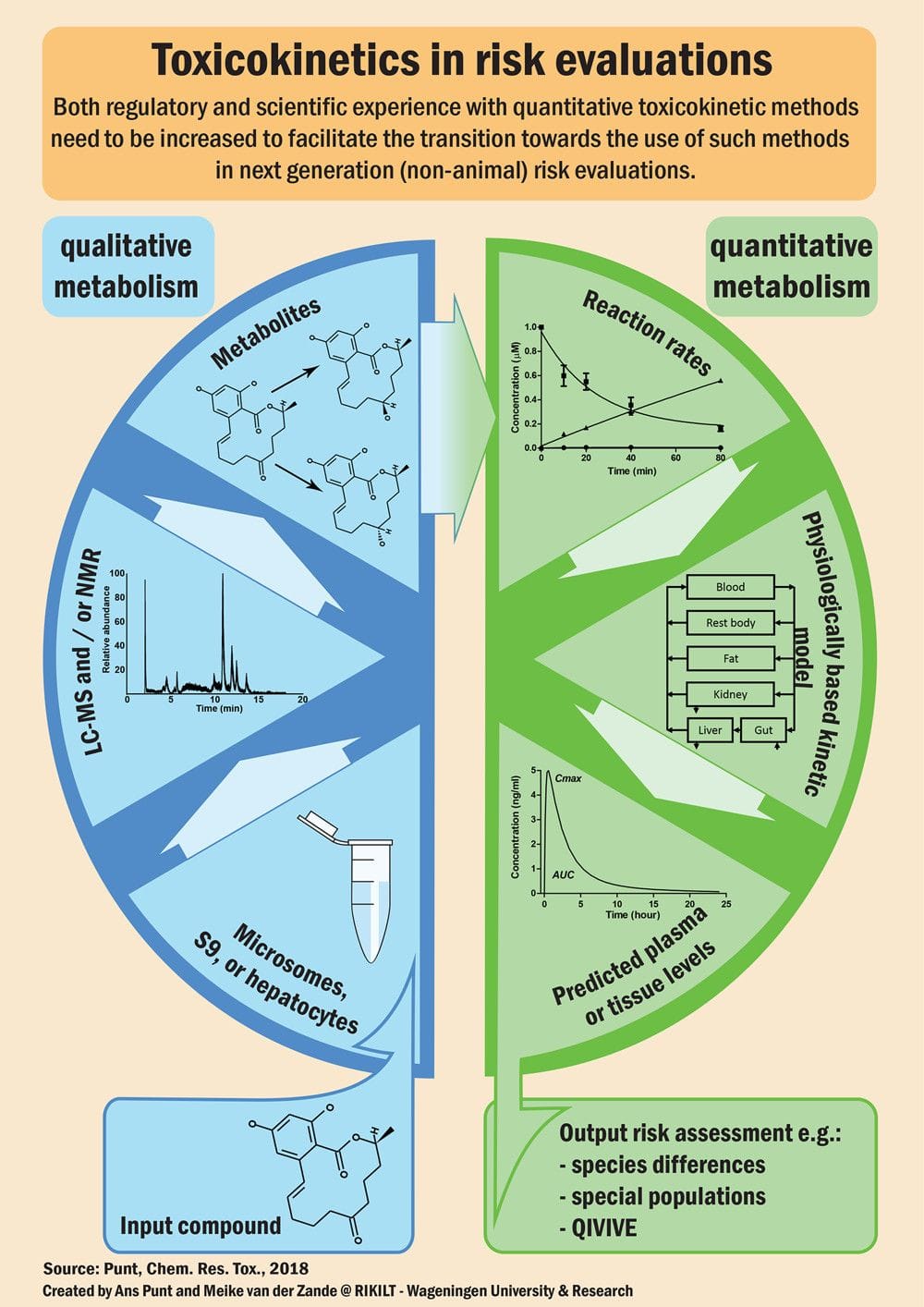
“Quantitative predictions of in vivo chemical levels based on in vitro data will become a cornerstone of next-generation nonanimal risk evaluations. Both regulatory and scientific experience with quantitative toxicokinetics must increase now for this transition to happen,” Punt writes in the abstract for “Toxicokinetics in Risk Evaluations,” a recent ToxWatch piece published in Chemical Research in Toxicology. The article explores the importance of having a quantitative picture of how a chemical is transformed and distributed in an organism to evaluate toxicity, complete with concrete examples.
“Only by increasing both regulatory and scientific experience with quantitative kinetics now can an ultimate transition toward the use of such methods in next generation (nonanimal) risk evaluations be made in the future,” she writes.
The poster from this article shows the cycle from the chemical structure of a compound through the qualitative and quantitative aspects of metabolism studies.
Click on the image below to get a high-resolution version of the poster.
Chemical Research in Toxicology has a new article type in 2018: ToxWatch, which pairs a single large, eye-catching graphic with short, accessible text to explore current issues in toxicology.
Chemical Research in Toxicology aims to serve the diverse, global research community with ToxWatch, just as it does in every article it publishes. To date, the journal has published ToxWatch from the U.S., Europe, and Asia. The journal welcomes ToxWatch contributions from all parts of the world.