Determining chemical makeup of drinking water is an increasingly fraught issue for many people in the U.S. and around the world. Figuring this out isn’t always easy or cheap. Today, scientists are reporting that they are using the familiar “coffee-ring effect” to analyze multiple components in a single drop of water easily, quickly and cheaply. […]

Determining chemical makeup of drinking water is an increasingly fraught issue for many people in the U.S. and around the world. Figuring this out isn’t always easy or cheap. Today, scientists are reporting that they are using the familiar “coffee-ring effect” to analyze multiple components in a single drop of water easily, quickly and cheaply. And someday, the public could use the method to test their own tap water.
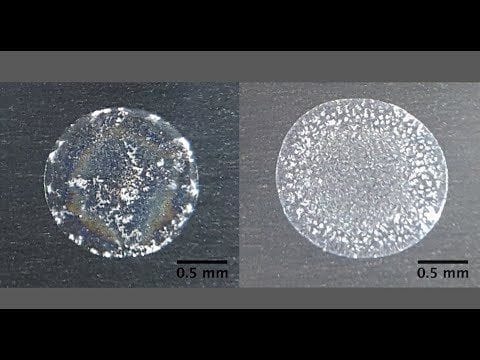
The coffee-ring effect is the familiar phenomenon in which the particles in a droplet of water tend to concentrate around the edges of the droplet as the water evaporates.
Learn more about this research:
Dr. Rebecca Lahr discussed her work at ACS 254th National Meeting & Exposition:
Lahr has been studying the coffee-ring effect since working on her Ph.D. thesis, originally using it to concentrate toxins in water for subsequent spectroscopic analysis. Now, she is applying it in her lab at Michigan State University to help the public learn more about the liquid coming from their taps. It turns out that the unique pattern left by a sample of water provides information on the content and properties of the water — dissolved solids, hardness, alkalinity and metal ions, for example. Determining each of these items in a sample traditionally required a different analytical method. The costs of those individual tests mount up.
To conduct the coffee-ring analysis, Lahr, graduate student Xiaoyan Li, and undergraduates Selett Allen and Alyssa Sanderson dried droplets of various tap water samples on low-cost aluminum substrates. Then, they photographed the resulting coffee-ring effect with a cell phone through an inexpensive jeweler’s loupe. After many experiments, they obtained reproducible residue patterns for tap waters from communities across southern Michigan. They also created synthetic tap water solutions to mimic community tap waters with varied amounts of hardness, alkalinity, sodium, chloride, sulfate, total dissolved solids, iron and copper.