Lithium-ion batteries can store large amounts of energy in small spaces, but overheating has been a key safety concern. Now, new research in Nano Letters offers a possible solution.

Batteries work by moving electrons from one electrode to the other in a circuit through an electronic device. But in a lithium-ion cell, the electrolyte liquid that separates these electrodes can evaporate when it overheats, causing a short circuit and subsequent chain of exothermic reactions. This makes these little powerhouses prone to catching fire or even exploding.
Overcharging and mechanical abuse can all increase the risk of damaging the internal stable state, resulting in a sharp rise in internal temperature and undesirable thermal runaway where multiple cells are chained together. At present, this means that lithium-ion batteries have a relatively limited range of acceptable temperatures and voltage for stable operation.
Previous work on external battery safety designs have included options for vents, sensors, or thermal fuses. However, the addition of external components adds weight, running counter to the preparation of ultralight and high-performance cells. Positive temperature coefficient materials are also a great idea, but have the drawbacks of low conductivity and large leakage current, thereby sacrificing battery performance.
Other groups have investigated the use of lithium-metal batteries rather than lithium-ion, and the possibility of fire-retardant electrolytes that can prevent the formation of deposits that encourage short circuits. But another route is a current-limiting switcher that is sensitive to internal temperature changes without additional weight or limitations on performance. With this in mind, a team of researchers in China set out to develop an internal safety technology that can put the brakes on a lithium-ion battery, quickly shutting it down when it gets too hot.
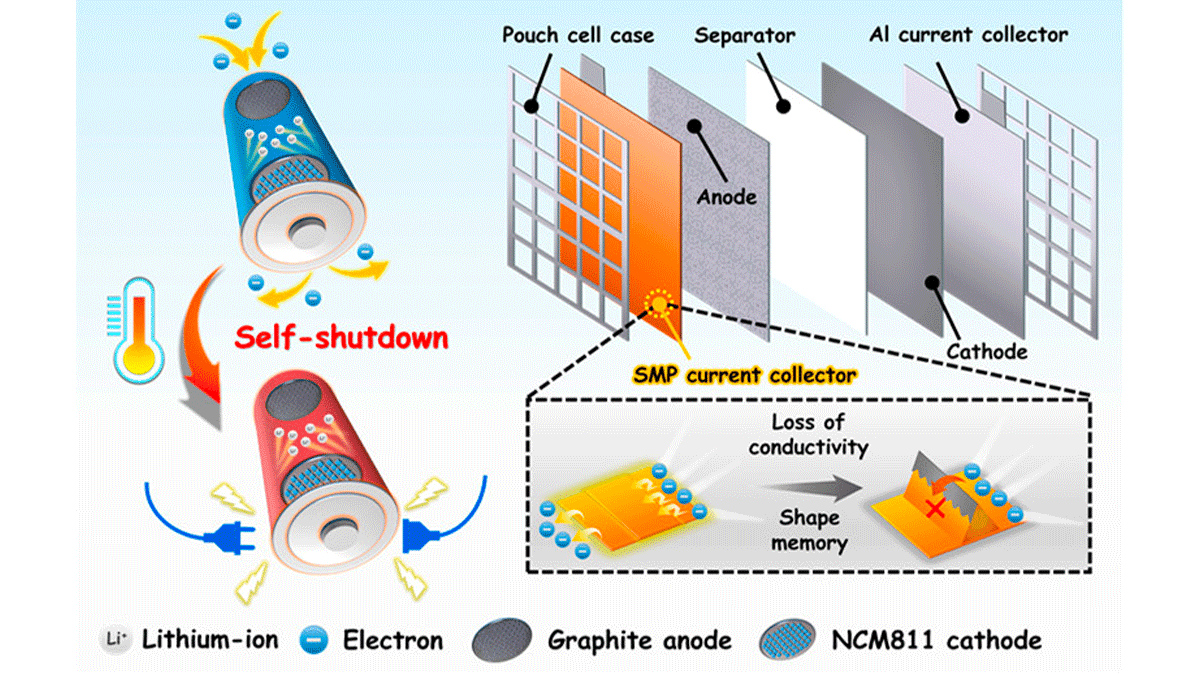
The authors used a thermally responsive memory polymer covered with conductive copper spray to create a material that would transmit electrons most of the time—with the ability to switch to "insulator mode" during overheating. This works based on a microscopic pattern programmed into the polymer, which appears only if the battery reaches temperatures above 197°F; this 3D pattern is then able to break apart the copper layer and stop the flow of electrons. Upon testing, the "on-to-off" response was extremely fast, with a complete disconnect time of fewer than 10 seconds.
Ultimately, this technology has the potential to make lithium-ion batteries much safer without having to sacrifice performance. But how likely are lithium-ion battery fires? Despite the risks, these are still rare events: industry experts put the figure at less than one fire per 10 million battery cells. However, lithium-ion batteries so widely used that there remains a need for reliable safety features to prevent these events from happening entirely.
Read the full article: Early Braking of Overwarmed Lithium-Ion Batteries by Shape-Memorized Current Collectors
Explore Related Research in ACS Journals
Correlating Kinetics to Cyclability Reveals Thermodynamic Origin of Lithium Anode Morphology in Liquid Electrolytes
David T. Boyle, Sang Cheol Kim, Solomon T. Oyakhire, Rafael A. Vilá, Zhuojun Huang, Philaphon Sayavong, Jian Qin, Zhenan Bao*, and Yi Cui*
DOI: 10.1021/jacs.2c08182
Chemically Induced Activity Recovery of Isolated Lithium in Anode-free Lithium Metal Batteries
Chengwei Ma, Suting Weng, Yuanxing Zhang, Xinyu Zhang, Tao Liu, Ling Liu, Zhiguang Zhao, Chengcai Liu, Zhikun Zhao, Xuefeng Wang, Borong Wu*, Daobin Mu*, and Feng Wu
DOI: 10.1021/acs.nanolett.2c02508
Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries
João C. Barbosa, Renato Gonçalves, Carlos M. Costa*, and Senentxu Lanceros-Méndez*
DOI: 10.1021/acsomega.2c01926
Lithium-Ion Solvation Structure in Organic Carbonate Electrolytes at Low Temperatures
Yeongseok Chae, Chaiho Lim, Jonggu Jeon, Minju Kim, Kyung-Koo Lee*, Kyungwon Kwak*, and Minhaeng Cho*
DOI: 10.1021/acs.jpclett.2c02106
Aqueous Binders for Cathodes: A Lodestar for Greener Lithium Ion Cells
Akhilash Mohanan Pillai, Patteth S. Salini, Bibin John*, and Mercy Thelakkattu Devassy
DOI: 10.1021/acs.energyfuels.2c00346
