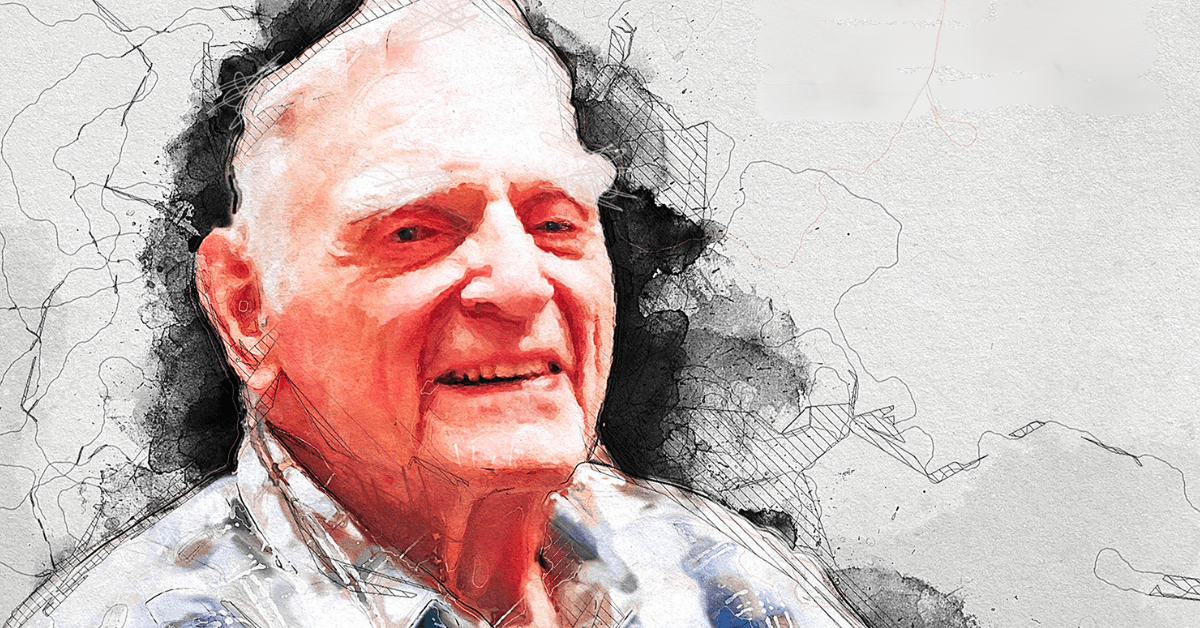
Professor John B. Goodenough, 2019 Nobel Laureate and pioneering innovator of lithium-ion battery development, has died at age 100. His contributions to the field have profoundly shaped our modern society—lithium-ion batteries have remained the world’s most reliable battery system for nearly half a century and have revolutionized our daily lives by paving the way for portable electronics and more viable renewable energy sources.
Prof. Goodenough leaves behind an extraordinary legacy of published work that spans 70 years, including a wealth of research articles contributed to ACS journals—he published his latest article in Chemistry of Materials at the age of 99. In remembrance of his impact and contributions to the field of chemistry, we present a selection of noteworthy research spanning Prof. Goodenough’s decades-long career as well as additional content highlighting his achievements throughout the years.
Remembering John B. Goodenough: Highlights from ACS Journals
Virtual Issue: John Goodenough at 100
Reviews, Perspectives, and Viewpoints
John Goodenough’s 100th Birthday Celebration: His Impact on Science and Humanity
DOI: 10.1021/acsenergylett.2c01343
Changing Outlook for Rechargeable Batteries
DOI: 10.1021/acscatal.6b03110
Perspective on Engineering Transition-Metal Oxides
DOI: 10.1021/cm402063u
The Li-Ion Rechargeable Battery: A Perspective
DOI: 10.1021/ja3091438
Challenges for Rechargeable Li Batteries
DOI: 10.1021/cm901452z
Research Articles
Interfacial Chemistry Enables Stable Cycling of All-Solid-State Li Metal Batteries at High Current Densities
DOI: 10.1021/jacs.1c00752
Upgrading Traditional Organic Electrolytes toward Future Lithium Metal Batteries: A Hierarchical Nano-SiO2-Supported Gel Polymer Electrolyte
DOI: 10.1021/acsenergylett.0c00412
Fast Li+ Conduction Mechanism and Interfacial Chemistry of a NASICON/Polymer Composite Electrolyte
DOI: 10.1021/jacs.9b12233
Li3N-Modified Garnet Electrolyte for All-Solid-State Lithium Metal Batteries Operated at 40 °C
DOI: 10.1021/acs.nanolett.8b03902
Garnet Electrolyte with an Ultralow Interfacial Resistance for Li-Metal Batteries
DOI: 10.1021/jacs.8b03106
Low-Cost High-Energy Potassium Cathode
DOI: 10.1021/jacs.6b12598
Electrochemical Nature of the Cathode Interface for a Solid-State Lithium-Ion Battery: Interface between LiCoO2 and Garnet-Li7La3Zr2O12
DOI: 10.1021/acs.chemmater.6b03870
Removal of Interstitial H2O in Hexacyanometallates for a Superior Cathode of a Sodium-Ion Battery
DOI: 10.1021/ja512383b
Estimating Hybridization of Transition Metal and Oxygen States in Perovskites from O K-edge X-ray Absorption Spectroscopy
DOI: 10.1021/jp410644j
Hollow Carbon-Nanotube/Carbon-Nanofiber Hybrid Anodes for Li-Ion Batteries
DOI: 10.1021/ja408421n
Evolution of Strategies for Modern Rechargeable Batteries
DOI: 10.1021/ar2002705
New Anode Framework for Rechargeable Lithium Batteries
DOI: 10.1021/cm200441h
Double-Perovskite Anode Materials Sr2MMoO6 (M = Co, Ni) for Solid Oxide Fuel Cells
DOI: 10.1021/cm8033643
Lithium Insertion into Transition-Metal Monosulfides: Tuning the Position of the Metal 4s Band
DOI: 10.1021/jp8038847
Surface protonation and electrochemical activity of oxides in aqueous solution
DOI: 10.1021/ja00162a006
Additional Highlights from ACS
Podcast: For John Goodenough’s 100th birthday, Stereo Chemistry revisits a fan-favorite interview with the renowned scientist – Chemical & Engineering News
Video: Lithium-ion batteries are (finally) Goodenough – Chemical & Engineering News
An Electrifying Choice for the 2019 Chemistry Nobel Prize: Goodenough, Whittingham, and Yoshino – Chemistry of Materials
Goodenough wins 2017 Welch Award – Chemical & Engineering News