Researchers have designed a taste-based sensor that releases flavor in response to influenza's viral enzyme, offering a simple, low-cost way to screen for infection.

Influenza has long been one of humanity's most persistent viral threats. Its pandemics have shaped history (the 1918 flu pandemic infected one in three people globally, killing more than World War I), and its seasonal surges continue to strain health systems worldwide.
Early detection and quarantine is critical for preventing outbreaks, but it remains difficult: influenza can be transmitted before symptoms emerge, and traditional tests are accurate but slow, expensive, and inaccessible for large-scale screening. There remains a need for first-line tools that are cost-effective, easy to distribute, and simple enough for anyone to use—ideally at the earliest signs of illness. Now, new work published in ACS Central Science explores a sensor that could allow us to easily diagnose influenza within the comfort of our homes, using just our sense of taste.
How the sensor works
The team focused on a viral enzyme called neuraminidase, which influenza must have to spread between cells. By designing molecules that respond specifically to this enzyme, the scientists developed a chemical system that turns a biological process—neuraminidase cleavage—into a sensory signal we can taste.
Their innovation centers on synthetic versions of N-acetylneuraminic acid, a sugar that neuraminidase naturally cuts during infection. The researchers attached a small flavor molecule, thymol (a terpenoid responsible for the flavor of thyme), to this sugar through a chemical bond that neuraminidase can break. When viral neuraminidase encounters the sensor molecule, it cleaves the bond and releases thymol, creating a detectable taste.
However, early versions of the sensor responded to both viral and bacterial neuraminidase. To achieve flu-specific detection, the team chemically fine-tuned the sugar molecule by adding methyl groups at two positions (O4 and O7). These subtle structural changes blocked bacterial enzymes but still allowed viral neuraminidase to act, creating a version of the sensor that only responds to the influenza enzyme.
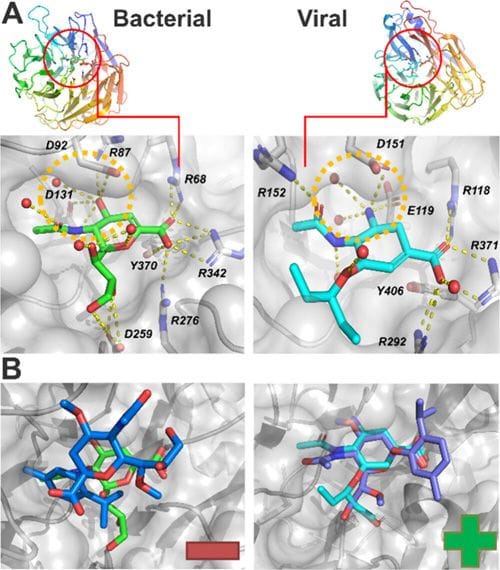
Proving selectivity and stability
Through a combination of synthetic chemistry, enzymatic assays, and molecular docking, the team demonstrated that this fine-tuned “α-sensor” selectively reacts to viral neuraminidase while remaining stable and non-toxic under normal conditions.
Tests in saliva showed that the enzyme levels found in patients with active influenza are high enough to trigger the reaction within about 30 minutes, while bacterial enzymes—and healthy saliva—leave the sensor untouched. The reaction is also inhibited by oseltamivir (Tamiflu), confirming that the sensor interacts directly with the same target the antiviral drug blocks.
Further experiments with live influenza virus (H1N1) produced measurable thymol release, reinforcing the system’s biological relevance. Structural modeling revealed why this selectivity works: influenza neuraminidase contains a broader active-site pocket than bacterial versions, allowing it to accommodate the methylated sugar structure, while bacterial neuraminidase cannot.
A taste-based test for the future
This concept opens the door to an entirely new class of diagnostics that use sensory chemistry instead of electronics or lab reagents. A person might one day be able to use a dissolvable strip, chewing gum, or lozenge, observing a distinct flavor if viral neuraminidase is present in their saliva.
The researchers estimate that only a few milligrams of the compound would be needed to produce a perceptible taste at typical enzyme concentrations found in infected saliva. One potential drawback is that taste can be dulled during infections, but this could be solved by replacing thymol with denatonium—a very bitter substance detectable at far lower concentrations—or a dye for visual detection.
As a first-line, low-cost screening tool, this could help people recognize infection early and seek confirmatory testing or isolation sooner. In large-scale outbreaks, these types of self-administered, accessible sensors could dramatically reduce the burden on healthcare systems. As the authors conclude in their publication, the human tongue could become "a 24/7 detector of influenza infection," a small but potentially transformative step toward making rapid diagnostics as simple as tasting your morning coffee.
Advances in influenza detection and treatment: explore related articles in ACS journals
A Finger-Actuated Microfluidic System for Point-Of-Care Detection of SARS-CoV-2 and Influenza A
Wu Zeng, Bao Li, Yanjing Chen, Baobao Lin, Xuanyu Gu, Peng Liu*, and Yan Zhang*
DOI: 10.1021/acs.analchem.5c01644
Development of a Universal Type A Influenza Viral RdRp-Induced Reporter System with Potential for Antiviral Drug Screening
Yuehong Chen, Wenguang Yang, Liangning Liao, Jing Li, Sen Zhang, Xiaoping Kang, Yuchang Li, Lu Zhao, Bao Dong, Huiyao Wang, Yi Hu*, Ye Feng*, and Tao Jiang*
DOI: 10.1021/acsinfecdis.4c00892
Needle-Free Transdermal Patch for Influenza Vaccination
Keisuke Tanaka, Hazuki Masaki, Kosuke Minamihata, Rie Wakabayashi, Yoshirou Kawaguchi, Noriho Kamiya, and Masahiro Goto*
DOI: 10.1021/acsabm.5c01361
Dual-Engineered Phage Vaccine Platform Facilitates STING Activation for Influenza Protection
Fei Wang, Shuhan Chen, Yinhe Xia, Caihong Liu, Zhou Xu, Ruilong Song, Wenfeng Liu, Tingting Liu*, Gang Chen*, and Qingquan Liu*
DOI: 10.1021/acsami.4c16246
Multilayer Adjuvanted Influenza Protein Nanoparticles Improve Intranasal Delivery and Antigen-Specific Immunity
Jaeyoung Park, Thomas Pho, Noopur Bhatnagar, Linh D. Mai, Mariela R. Rodriguez-Otero, Surya Sekhar Pal, Chau Thuy Tien Le, Sarah E. Jenison, Chenyu Li, Grace A. May, Marisa Arioka, Sang-Moo Kang*, and Julie A. Champion*
DOI: 10.1021/acsnano.4c14735
Single-Dose Oral Influenza Antiviral Prodrug Enabled by Cholesterol Conjugation
Chenning Li, Xun Lv*, Chenxi Cheng, Shuihong Cheng, Yiran Li, Yuhai Bi, Lifeng Fu, George Fu Gao*, and Xuebing Li*
DOI: 10.1021/acs.jmedchem.5c01808
Development Strategies for Influenza Vaccines Utilizing Phage RNA Polymerase and Capping Enzyme NP868R
Weijun Wang, Zihan Ma, Qiuli Lou, Tingting Li, Zhaoying Huang, Wen Yin, Chunbo Lou*, and Yanhui Xiang*
DOI: 10.1021/cbe.5c00030
