Opioid use has become a big issue. Existing treatments can reverse overdoses, but current barriers to access are no good when time is of the essence. Now, new stimuli-responsive materials could provide temporal control of drug delivery.
Every year in the United States, more than 40,000 people die from an opioid overdose.1 Antagonists such as naloxone can save lives if they are used in time, but effective administration during an overdose requires that bystanders are familiar with, have access to, and know how to use these antidotes. Additionally, many overdose deaths occur among those using drugs alone.2
Previous work has focused on long-acting formulations by harnessing covalent nanoparticle drug-delivery technology. This approach was able to provide sustained linear release of naloxone, and it blocked the effects of high-dose acute morphine for up to 98 hours.3 But an embedded system that releases naloxone only when needed would be a major advance.
To address this, a team at Harvard Medical School designed a light-responsive polymer–naloxone conjugate, formulated in injectable nanoparticles.4 The research, published in Nano Letters, describes experiments with the nanoparticles, and their ability to reverse the effect of morphine in mice. The key breakthrough was that the naloxone was released when irradiated with blue light—but only that of a specific wavelength and with an intensity greater than ambient light.
The idea in practice would be that the depot would be injected subcutaneously by healthcare practitioners, and would subsequently be on the patient’s person for immediate use in a future emergency situation. In the mouse model, the nanoparticles did not show trigger opioid reversal if irradiation was performed 2 months after injection, suggesting that repeated dosing might be needed in the clinic.
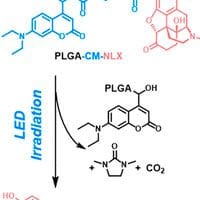
On-Demand Opioid Effect Reversal with an Injectable Light-Triggered Polymer-Naloxone Conjugate
DOI: 10.1021/acs.nanolett.3c03426
In addition to opioid reversal, this kind of on-demand system would also be beneficial for pain relief—but there are challenges in achieving stimulus sensitivity, enhancing depth of tissue penetration, and determining successful repeatability of triggers.5
Light has been used before to trigger the timing, intensity, and duration of on-demand local anesthesia, adjusted by modulating the intensity and/or duration of irradiation. Other work by members of the team has looked at liposomes that change membrane permeability on exposure to near-UV light, thereby releasing their contents.6 These nanoparticulate systems could selectively target any tissue upon illumination.
The interest in targeted and triggered nanomaterials in the medicinal field continues to increase. Some passive systems currently in development rely on endogenous environmental properties such as pH, hypoxia, or enzyme activity to provide the stimulus that causes the drug delivery event, whereas others harness a more active triggering event such as light, ultrasound, or chemicals.7
For opioid users, the ability to control drug in real-time is hugely beneficial—both for pain relief and overdose reversal. But this could also translate into diseases such as cancer, where the therapeutic effect of drugs is limited by toxicity. More research is needed to further this potential, but this could be an exciting prospect—and a solution that saves many lives.
References:
- Florence, C. et al. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol Depend. 2021, 218, 108350.
- Deo, V. S. et al. The Need to Rethink Harm Reduction for People Using Drugs Alone to Reduce Overdose Fatalities. Subst. Use Misuse. 2023, 15, 1–9.
- Kassick, A. J. et al. Covalent Poly(lactic acid) Nanoparticles for the Sustained Delivery of Naloxone. ACS Appl. Bio Mater. 2019, 2, 8, 3418–3428.
- Zhang, W. et al. On-Demand Opioid Effect Reversal with an Injectable Light-Triggered Polymer-Naloxone Conjugate. Nano Lett. 2023, 23, 22, 10545–10553.
- Rwei, A. Y. et al. Enhanced Triggering of Local Anesthetic Particles by Photosensitization and Photothermal Effect Using a Common Wavelength. Nano Lett. 2017, 17, 11, 7138–7145.
- Dvir, T. et al. Photo-Targeted Nanoparticles. Nano Lett. 2010, 10, 1, 250–254.
- Zhang, W. and Kohane, D. S. Keeping Nanomedicine on Target. Nano Lett. 2021, 21, 1, 3–5.
