Browse the most-read organic/inorganic chemistry articles 2016, including research, reviews, perspectives, and editorial pieces.

There are lots of different ways to look at the reach of an article. You can look at citations, Altmetric Attention Scores, awards, and more. One way to consider the influence of an article is just by looking at how many people chose to read it. To that end, we’ve compiled lists of the five most-read chemistry articles that appeared in each ACS Publications journal in 2016, including research, reviews, perspectives and editorial pieces. These lists were not chosen by the journal’s editors and should not be taken as a “best of” list for 2016, but rather as an interesting perspective on where the chemistry community allocated their attention over the past year.
Of course, certain factors such as publishing earlier in the year, being featured in a special issue or being made open access may influence which items appeared on this list. To that end, ACS Axial will be publishing monthly updates on the most read chemistry articles of 2017 all year long. Don’t see your favorite papers on the list? Feel free to share your picks in the comments below.
Read more most-read articles: Analytical | Applied | Biological | Materials Science & Engineering | Physical
***
Cobalt-Catalyzed C–H Activation
ACS Catal., 2016, 6 (2), pp 498–525
DOI: 10.1021/acscatal.5b02344
Toward Benchmarking in Catalysis Science: Best Practices, Challenges, and Opportunities
Open Access Through ACS Editors’ Choice
ACS Catal., 2016, 6 (4), pp 2590–2602
DOI: 10.1021/acscatal.6b00183
Metal-Catalyzed Carboxylation of Organic (Pseudo)halides with CO2
ACS Catal., 2016, 6 (10), pp 6739–6749
DOI: 10.1021/acscatal.6b02124
Catalytic Methods for Aromatic C–H Amination: An Ideal Strategy for Nitrogen-Based Functional Molecules
ACS Catal., 2016, 6 (2), pp 610–633
DOI: 10.1021/acscatal.5b02417
Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis
ACS Catal., 2016, 6 (9), pp 5887–5903
DOI: 10.1021/acscatal.6b01222
***
ACS Combinatorial Science
CuAAC: An Efficient Click Chemistry Reaction on Solid Phase
ACS Comb. Sci., 2016, 18 (1), pp 1–14
DOI: 10.1021/acscombsci.5b00087
Discovery of a Direct Ras Inhibitor by Screening a Combinatorial Library of Cell-Permeable Bicyclic Peptides
Open Access Through ACS Editors’ Choice
ACS Comb. Sci., 2016, 18 (1), pp 75–85
DOI: 10.1021/acscombsci.5b00164
Rapid Discovery of Functional Small Molecule Ligands against Proteomic Targets through Library-Against-Library Screening
Open Access Through ACS Editors’ Choice
Direct Phenotypic Screening in Mice: Identification of Individual, Novel Antinociceptive Compounds from a Library of 734 821 Pyrrolidine Bis-piperazines
Open Access Through ACS Editors’ Choice
ACS Comb. Sci., 2016, 18 (1), pp 51–64
DOI: 10.1021/acscombsci.5b00126
Simulated Screens of DNA Encoded Libraries: The Potential Influence of Chemical Synthesis Fidelity on Interpretation of Structure–Activity Relationships
ACS Comb. Sci., 2016, 18 (7), pp 415–424
DOI: 10.1021/acscombsci.6b00001
***
ACS Medicinal Chemistry Letters
Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and mTOR
Open Access Through ACS Editors’ Choice
ACS Med. Chem. Lett., 2016, 7 (4), pp 351–356
DOI: 10.1021/acsmedchemlett.6b00005
Hope and Disappointment: Covalent Inhibitors to Overcome Drug Resistance in Non-Small Cell Lung Cancer
ACS Med. Chem. Lett., 2016, 7 (1), pp 2–5
DOI: 10.1021/acsmedchemlett.5b00475
Optimization of Tubulysin Antibody–Drug Conjugates: A Case Study in Addressing ADC Metabolism
Open Access Through ACS Editors’ Choice
ACS Med. Chem. Lett., 2016, 7 (11), pp 977–982
DOI: 10.1021/acsmedchemlett.6b00195
High Drug Prices Hurt Everyone
ACS Med. Chem. Lett., 2016, 7 (6), pp 544–546
DOI: 10.1021/acsmedchemlett.6b00139
GSK6853, a Chemical Probe for Inhibition of the BRPF1 Bromodomain
Open Access Through ACS Editors’ Choice
ACS Med. Chem. Lett., 2016, 7 (6), pp 552–557
DOI: 10.1021/acsmedchemlett.6b00092
***
Simple Method To Prepare Oligonucleotide-Conjugated Antibodies and Its Application in Multiplex Protein Detection in Single Cells
Open Access Through ACS AuthorChoice
Bioconjugate Chem., 2016, 27 (1), pp 217–225
DOI: 10.1021/acs.bioconjchem.5b00613
Microwave-Triggered Smart Drug Release from Liposomes Co-encapsulating Doxorubicin and Salt for Local Combined Hyperthermia and Chemotherapy of Cancer
Open Access Through ACS Editors’ Choice
Bioconjugate Chem., 2016, 27 (12), pp 2931–2942
DOI: 10.1021/acs.bioconjchem.6b00603
18F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels–Alder Click Chemistry
Open Access Through ACS AuthorChoice
Bioconjugate Chem., 2016, 27 (2), pp 298–301
DOI: 10.1021/acs.bioconjchem.5b00504
New Chelators for Low Temperature Al18F-Labeling of Biomolecules
Open Access Through ACS Editors’ Choice
Bioconjugate Chem., 2016, 27 (3), pp 790–798
DOI: 10.1021/acs.bioconjchem.6b00012
NanoLuc: A Small Luciferase Is Brightening Up the Field of Bioluminescence
Bioconjugate Chem., 2016, 27 (5), pp 1175–1187
DOI: 10.1021/acs.bioconjchem.6b00112
***f
Inorganic Chemistry
Hydrogen Evolution from Water under Aerobic Conditions Catalyzed by a Cobalt ATCUN Metallopeptide
Open Access Through ACS Editors’ Choice
Inorg. Chem., 2016, 55 (4), pp 1355–1357
DOI: 10.1021/acs.inorgchem.5b02157
Lead-Free MA2CuClxBr4–x Hybrid Perovskites
Inorg. Chem., 2016, 55 (3), pp 1044–1052
DOI: 10.1021/acs.inorgchem.5b01896
Heterobimetallic N-Heterocyclic Carbene Complexes: A Synthetic, Spectroscopic, and Theoretical Study
Open Access Through ACS Editors’ Choice
Inorg. Chem., 2016, 55 (14), pp 6882–6891
DOI: 10.1021/acs.inorgchem.6b00222
Homogeneous Photocatalytic Water Oxidation with a Dinuclear CoIII–Pyridylmethylamine Complex
Inorg. Chem., 2016, 55 (3), pp 1154–1164
DOI: 10.1021/acs.inorgchem.5b02336
UiO-67-type Metal–Organic Frameworks with Enhanced Water Stability and Methane Adsorption Capacity
Inorg. Chem., 2016, 55 (5), pp 1986–1991
DOI: 10.1021/acs.inorgchem.5b02257
***
Journal of Medicinal Chemistry
Novel Cephalosporins Selectively Active on Nonreplicating Mycobacterium tuberculosis
Open Access Through ACS Editors’ Choice

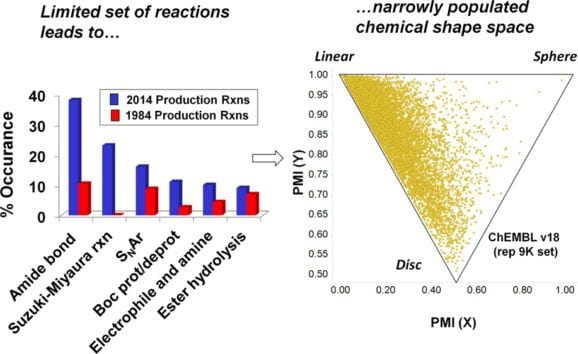
Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?
Open Access Through ACS AuthorChoice
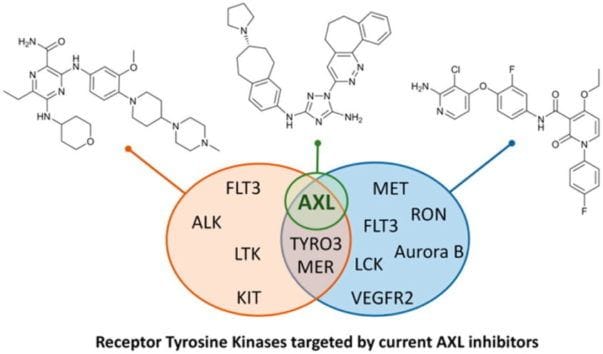
AXL Inhibitors in Cancer: A Medicinal Chemistry Perspective
The Medicinal Chemistry of Dengue Virus
Open Access Through ACS Editors’ Choice

A Real-World Perspective on Molecular Design
This article is part of the Computational Methods for Medicinal Chemistry special issue.
***
Natural Products as Sources of New Drugs from 1981 to 2014
Open Access Through ACS Editors’ Choice
J. Nat. Prod., 2016, 79 (3), pp 629–661
DOI: 10.1021/acs.jnatprod.5b01055
Affinity Crystallography: A New Approach to Extracting High-Affinity Enzyme Inhibitors from Natural Extracts
Open Access Through ACS Editors’ Choice
J. Nat. Prod., 2016, 79 (8), pp 1962–1970
DOI: 10.1021/acs.jnatprod.6b00215
Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS)
Open Access Through ACS Editors’ Choice
J. Nat. Prod., 2016, 79 (3), pp 616–628
DOI: 10.1021/acs.jnatprod.5b00947
LC-MS- and 1H NMR-Based Metabolomic Analysis and in Vitro Toxicological Assessment of 43 Aristolochia Species
Open Access Through ACS Editors’ Choice
J. Nat. Prod., 2016, 79 (1), pp 30–37
DOI: 10.1021/acs.jnatprod.5b00556
Chemodiversity of Ladder-Frame Prymnesin Polyethers in Prymnesium parvum
Open Access Through ACS Editors’ Choice
J. Nat. Prod., 2016, 79 (9), pp 2250–2256
DOI: 10.1021/acs.jnatprod.6b00345
***
Photoredox Catalysis in Organic Chemistry
Open Access Through ACS Editors’ Choice
J. Org. Chem., 2016, 81 (16), pp 6898–6926
DOI: 10.1021/acs.joc.6b01449
Recent Advances in C–H Functionalization
J. Org. Chem., 2016, 81 (2), pp 343–350
DOI: 10.1021/acs.joc.5b02818
Total Synthesis of a Diacetonide Derivative of Thuggacin A
J. Org. Chem., 2016, 81 (5), pp 1786–1797
DOI: 10.1021/acs.joc.5b02426
Chan–Evans–Lam Amination of Boronic Acid Pinacol (BPin) Esters: Overcoming the Aryl Amine Problem
J. Org. Chem., 2016, 81 (9), pp 3942–3950
DOI: 10.1021/acs.joc.6b00466
Palladium-Catalyzed Double-Suzuki–Miyaura Reactions Using Cyclic Dibenziodoniums: Synthesis of o-Tetraaryls
Open Access Through ACS Editors’ Choice
J. Org. Chem., 2016, 81 (4), pp 1317–1323
DOI: 10.1021/acs.joc.5b02255
***
Molecular Pharmaceutics
Deep Learning Applications for Predicting Pharmacological Properties of Drugs and Drug Repurposing Using Transcriptomic Data
Open Access Through ACS Editors’ Choice

Applications of Deep Learning in Biomedicine

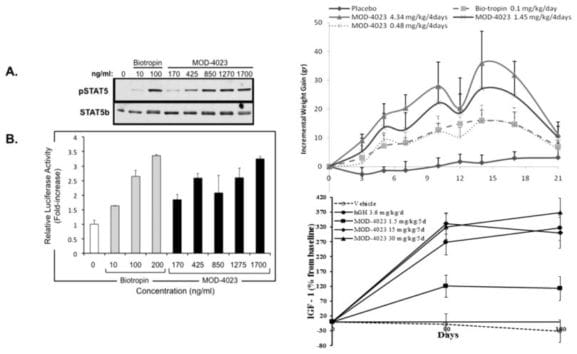
In Vitro and in Vivo Characterization of MOD-4023, a Long-Acting Carboxy-Terminal Peptide (CTP)-Modified Human Growth Hormone
Open Access Through ACS Editors’ Choice
Light Control of Insulin Release and Blood Glucose Using an Injectable Photoactivated Depot
Open Access Through ACS Editors’ Choice
Mol. Pharmaceutics, 2016, 13 (11), pp 3835–3841
DOI: 10.1021/acs.molpharmaceut.6b00633
Molecular Imaging of Pancreatic Cancer with Antibodies
Open Access Through ACS AuthorChoice
Mol. Pharmaceutics, 2016, 13 (1), pp 8–24
DOI: 10.1021/acs.molpharmaceut.5b00626
***
Darwinolide, a New Diterpene Scaffold That Inhibits Methicillin-Resistant
Open Access Through ACS Editors’ Choice
Org. Lett., 2016, 18 (11), pp 2596–2599
DOI: 10.1021/acs.orglett.6b00979
Time Economical Total Synthesis of (−)-Oseltamivir
Org. Lett., 2016, 18 (14), pp 3426–3429
DOI: 10.1021/acs.orglett.6b01595
Total Synthesis of (+)-Vicenin-2
Open Access Through ACS Editors’ Choice
Org. Lett., 2016, 18 (18), pp 4488–4490
DOI: 10.1021/acs.orglett.6b02203
Total Synthesis of Lycopalhine A
Org. Lett., 2016, 18 (6), pp 1494–1496
DOI: 10.1021/acs.orglett.6b00338
Total Synthesis of (±)-Gracilioether F
Org. Lett., 2016, 18 (5), pp 1032–1035
DOI: 10.1021/acs.orglett.6b00161
***
Organic Process Research & Development
NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry
(Open Access Through ACS Editors’ Choice)
Org. Process Res. Dev., 2016, 20 (3), pp 661–667
DOI: 10.1021/acs.oprd.5b00417
Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals
Org. Process Res. Dev., 2016, 20 (2), pp 140–177
DOI: 10.1021/op500305s
Vacuum Desiccator as a Simple, Robust, and Inexpensive NMR Tube Cleaner
Org. Process Res. Dev., 2016, 20 (2), pp 319–319
DOI: 10.1021/acs.oprd.6b00001
Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products
(Open Access Through ACS Editors’ Choice)
Org. Process Res. Dev., 2016, 20 (1), pp 2–25
DOI: 10.1021/acs.oprd.5b00325
Toward a More Holistic Framework for Solvent Selection
(Open Access Through ACS Editors’ Choice)
Org. Process Res. Dev., 2016, 20 (4), pp 760–773
DOI: 10.1021/acs.oprd.6b00015
***
An Editorial About Elemental Analysis
Organometallics, 2016, 35 (19), pp 3255–3256
DOI: 10.1021/acs.organomet.6b00720
A Frustrated Lewis Pair Based on a Cationic Aluminum Complex and Triphenylphosphine
(Open Access Through ACS Editors’ Choice)
Organometallics, 2016, 35 (2), pp 207–217
DOI: 10.1021/acs.organomet.5b00927
A Transmetalation Reaction Enables the Synthesis of [18F]5-Fluorouracil from [18F]Fluoride for Human PET Imaging
(Open Access Through ACS Editors’ Choice)
Organometallics, 2016, 35 (7), pp 1008–1014
DOI: 10.1021/acs.organomet.6b00059
Pyramidanes: The Covalent Form of the Ionic Compounds
(Open Access Through ACS Editors’ Choice)
Organometallics, 2016, 35 (3), pp 346–356
DOI: 10.1021/acs.organomet.5b00924
Legacy of Richard Heck
Organometallics, 2016, 35 (9), pp 1177–1178
DOI: 10.1021/acs.organomet.6b00261
***
Read more most-read articles: Analytical | Applied | Biological | Materials Science & Engineering | Physical
