A recent study reveals how a myriad of nonterpenoid compounds play a pivotal role in creating the distinctive citrus-to-savory scents of Cannabis sativa L. Unearth the chemistry behind these unique aromas and how they redefine our understanding of cannabis scent profiles.

With regulation and legalization of cannabis in many countries, the market is poised for significant growth. But there remains a need to understand this plant and its diverse uses. There are different varieties of Cannabis—from those with a high tetrahydrocannabinol (THC) content, which are consumed for their intoxicating psychoactive effects, to cultivars with less than 0.3% THC, primarily grown for medicinal cannabidiol (CBD) and the textiles fiber industry (hemp).1
Cannabis produces a myriad of volatile metabolites that contribute to its unique smell. The major volatile constituents include around 200 different terpenes and their oxygenated derivates.2 Terpenes volatilize easily under ambient conditions, and as such are regarded as important aroma compounds in nature—so much so that chemotaxonomic classification schemes and legal product labelling already make use of myrcene, limonene, caryophyllene, and terpinolene—and these compounds are often reported in and around cannabis cultivation facilities.1
It seems counterintuitive that classification should be needed beyond the binomial scientific names of C.indica or C.sativa, but modern cannabis is in fact more often a hybrid of both and can have unexpected phenotypic, aromatic, or chemical characteristics. It is therefore possible that different volatiles traditionally thought of as flavorants also contribute to the characteristic smell and may help to broadly differentiate varieties.
New research published in ACS Omega shows that terpene expression is pretty similar across varieties, suggesting these chemicals might not actually make that much of a contribution to specific scents.3 Instead, the team found that many of the minor, non-terpenoid compounds correlate with sweet or savory aromas. The study examined the chemical profiles of 31 cannabis ice hash rosin extracts using 2D gas chromatography, time-of-flight mass spectrometry, and flame ionization detection. When coupled with sensory studies, the researchers were able to derive correlations between compounds that produce diverse scents.
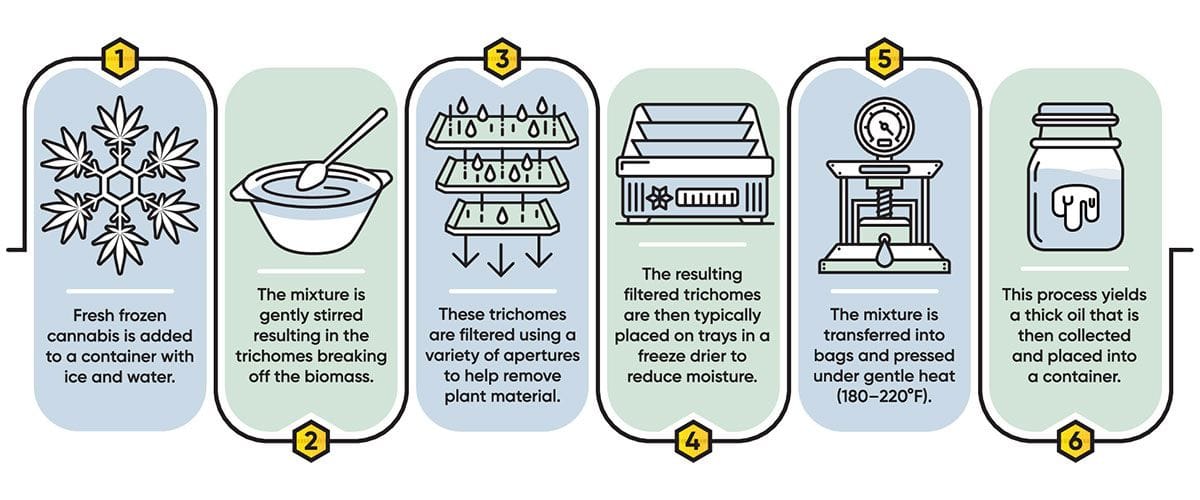
Across the spectrum, cannabis aromas were defined in three main categories:
- Sweet at one end of the spectrum (encompassing fruity or tropical notes);
- Savory at the other (onion, garlic, or chemical);
- Prototypical aromas in the middle (earthy, spicy, or skunky).
Each of these categories correlated with different compounds. For example, esters are found in nearly every fruit and contribute strongly to aroma and flavors. The new research identified 30 esters in cannabis, each with different aromatic characteristics—even within a single variety. Two compounds of note include ethyl hexanoate and n-propyl hexanoate: one a fruity, apple-like aroma, and the other a scent of blackberry and pineapple.
Another key finding was the identification of a new class of volatile sulfur compounds (VSCs) containing the 3-mercaptohexyl functional group responsible for citrus aromas, and skatole (3-methylindole) as the source of a chemical aroma in others.
Previous work using different investigative strategies has also identified a new family of prenylated VSCs in cannabis, of which 3-methyl-2-butene-1-thiol was shown to be responsible for the characteristic "skunk" odor.4-5
There may be questions beyond odor impact, such as toxicology or atmospheric chemistry of emissions, but for now, the authors argue that these results contribute to a richer understanding of cannabis chemistry—and crucially highlight how the role of terpenes in aroma profiles may not be as pungent as we previously thought.
References
- de Ferreyro Monticelli, D. et al. Cannabis Cultivation Facilities: A Review of Their Air Quality Impacts from the Occupational to Community Scale. Environ. Sci. Technol. 2022, 56, 5, 2880–2896.
- Shapira, A. et al. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019, 91, 17, 11425–11432.
- Oswald, I. W. H. et al. Minor, Nonterpenoid Volatile Compounds Drive the Aroma Differences of Exotic Cannabis. ACS Omega 2023, 8, 42, 39203–39216.
- Oswold, I. W. H. et al. Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography. ACS Omega 2021, 6, 47, 31667–31676.
- Koziel, J. A. et al. “Skunky” Cannabis: Environmental Odor Troubleshooting and the “Need-for-Speed". ACS Omega 2022, 7, 23, 19043–19047.
