Chemical reactions occurring in space ice have the potential to make important molecules for life without needing high-energy radiation. Studying how these molecules form in space can help us learn more about their role in the origin of life on Earth.

Topics don’t get much bigger than the beginnings of life, and although theories abound, there are significant gaps in our knowledge around where and how key molecules formed. For example, it has long been hypothesized that one of the building blocks for life, amino acids, could have formed during reactions in the “primordial soup” of the early prebiotic Earth. Other groups suggest that amino acids were carried to the Earth’s surface by meteorites, but this raises the question of where they seeded before making the trip.
In new work published in ACS Central Science, researchers have revealed new insights into how certain chemical reactions happen in space. They focused on mixtures of carbon dioxide and ammonia, similar to what you'd find in the icy regions around new stars and planets. Using Fourier transform infrared spectroscopy, they looked at the lowest temperatures needed to start these reactions.
What they found was interesting: when these mixtures were in an icy state, a chemical called carbamic acid formed and broke down more quickly. But once the ice turned into gas (a process called sublimation), the carbamic acid became much more stable and didn't break apart as easily. This was further confirmed using a highly sensitive method called photoionization reflectron time-of-flight mass spectrometry, which showed that carbamic acid can survive as a gas even after being formed under different conditions.
This is important because carbamic acid can break down further into amino acids. The researchers observed that even at temperatures as high as 290 K (which is a bit warmer than the cold regions where planets form), a form of carbamic acid was stable. This suggests that such molecules could survive in the early stages of star and planet formation.
The study concludes that it's quite possible for these stable molecules to have reached the early Earth via meteorites and comets, potentially bringing with them the ingredients necessary for life.
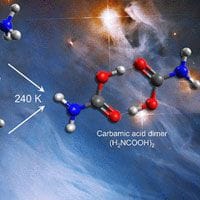
Thermal Synthesis of Carbamic Acid and Its Dimer in Interstellar Ices: A Reservoir of Interstellar Amino Acids
DOI: 10.1021/acscentsci.3c01108
Previously, members of the same research group used the same reflectron time-of-flight mass spectrometry technique to identify the elusive N-hydroxyoxaziridine (c-H2CON(OH)), a chiral, high energy isomer of nitromethane. This molecule is among the simplest representatives of oxaziridines, a class of molecules known for their unique chemical and physical properties due to the presence of electronegative atoms within a strained three-membered ring.
N. Fabian Kleimeier and Ralf I. Kaiser also reported the first bottom-up preparation of 1,1-ethenediol (H2CC(OH)2)—the simplest unsaturated geminal enol of acetic acid and potential precursor for the formation of glycine—in interstellar analogue ices of carbon dioxide and methane processed by proxies of galactic cosmic rays.3 This work, published in The Journal of Physical Chemistry Letters, provides critical insights into the chemistry of interstellar ices and the formation of complex organic molecules in space.
Together, these studies provide compelling evidence that key molecules such as amino acids and hydrocarbons can be synthesized in extreme environments—from low-temperature molecular clouds and hydrocarbon-rich atmospheres of planets and moons, to high-temperature environments like circumstellar envelopes of carbon-rich stars and combustion systems.4 These findings all shed light on the universe in which we live, and the mechanisms that drive stellar and biological processes.
References
- Marks, J. H. et al. Thermal Synthesis of Carbamic Acid and Its Dimer in Interstellar Ices: A Reservoir of Interstellar Amino Acids. ACS Cent. Sci. 2023, 9, 12, 2241–2250.
- Singh, S. K. et al. Gas Phase Identification of the Elusive N-Hydroxyoxaziridine (c-H2CON(OH)): A Chiral Molecule. J. Phys. Chem. Lett. 2020, 11, 13, 5383–5389.
- Kleimeier, N.F. and Kaiser, R. I. Bottom-Up Synthesis of 1,1-Ethenediol (H2CC(OH)2)─The Simplest Unsaturated Geminal Diol─In Interstellar Analogue Ices. J. Phys. Chem. Lett. 2022, 13, 1, 229–235.
- Kaiser, R. I. and Hansen, N. An Aromatic Universe–A Physical Chemistry Perspective. J. Phys. Chem. A 2021, 125, 18, 3826–3840.
