Learn how supercapacitors, unlike lithium-ion batteries, charge quickly, deliver high power, and are more stable and long-lasting. Although current models have low energy density, new "candy cane" supercapacitors show promising improvements.

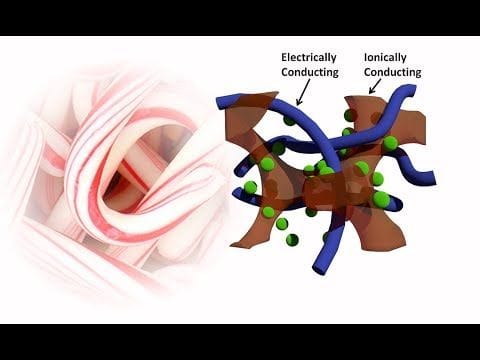
Batteries, specifically lithium-ion batteries, dominate the energy storage landscape. However, the chemical reactions underlying the charging and discharging process in batteries are slow, limiting how much power they can deliver. Plus, batteries tend to degrade over time, requiring replacement. An alternate energy storage device, the supercapacitor, charges rapidly and generates serious power, which could potentially allow electric cars to accelerate more quickly, among other applications. Plus, supercapacitors store energy electrostatically, not chemically, which makes them more stable and long-lasting than many batteries. But today’s commercially available supercapacitors require binders and have a low energy density, limiting their application in emerging go-anywhere electronics.
Tiesheng Wang, a graduate student in the lab of Stoyan Smoukov, Ph.D., at the University of Cambridge (U.K.) suspected that a flexible conducting polymer-based material from another project they were working on could be a better alternative. Conducting polymers, such as poly(3,4-ethylenedioxythiophene) (PEDOT), are candidate supercapacitors that have advantages over traditional carbon-based supercapacitors as charge storage materials. They are pseudocapacitive, meaning they allow reversible electrochemical reactions, and they also are chemically stable and inexpensive. However, ions can only penetrate the polymers a couple of nanometers deep, leaving much of the material as dead weight. Scientists working to improve ion mobility had previously developed nanostructures that deposit thin layers of conducting polymers on top of support materials, which improves supercapacitor performance by making more of the polymer accessible to the ions. The drawback, according to Wang, is that these nanostructures can be fragile, difficult to make reproducibly when scaled-up and poor in electrochemical stability, limiting their applicability.
Watch Tiesheng Wang Explain His Research at the 255th ACS National Meeting & Exposition:
When tested, the candy cane supercapacitor demonstrated improvements over PEDOT alone with regard to flexibility and cycling stability. It also had nearly double the specific capacitance compared to conventional PEDOT-based supercapacitors.