In 2019, UNESCO is celebrating the International Year of the Periodic Table of Chemical Elements, in honor of the 150th anniversary of Dmitry Mendeleev’s version of the periodic table. ACS has been joining in the fun all year long and now The Journal of Physical Chemistry A, B, C, and Letters (JPC) are getting in on […]

In 2019, UNESCO is celebrating the International Year of the Periodic Table of Chemical Elements, in honor of the 150th anniversary of Dmitry Mendeleev’s version of the periodic table. ACS has been joining in the fun all year long and now The Journal of Physical Chemistry A, B, C, and Letters (JPC) are getting in on the celebration with a collection of articles about each of the elements from across the journal’s 123-year history.
“There are numerous interesting stories that are woven through these papers. We could easily write a story about each element,” the JPC Editorial Team writes in an editorial for the issue.
The articles below are organized by the period of the element they represent. Each article’s listing is accompanied by commentary from one of the journal’s editors. While the periodic table contains 118 elements, this list only represents 116 of them, since the journal has yet to publish a paper focusing on elements 109 and 110, Meitnerium and Darmstadtium.
If you want to be the first to publish work in the journal on those elements, check out the journal’s submission guidelines.
Click Below to Discover Articles for the Elements in Each Period
(AN#37) – Rb – Rubidium

Cesium and Rubidium Ion Equilibriums in Illite Clay
J. Phys. Chem., 1983, 87 (7), pp 1213–1219
DOI: 10.1021/j100230a024
Rubidium cations in solutions exhibit similar properties as the alkali cations in neighboring rows of the periodic table, which makes it a challenge to investigate the corresponding ion exchange processes in illite clays —JPC Senior Editor Pavel Jungwirth
***
(AN#38) – Sr – Strontium

Nature of Deficiency in Nonstoichiometric Hydroxyapatites. I. Catalytic Activity of Calcium and Strontium Hydroxyapatites
J. Phys. Chem., 1971, 75 (20), pp 3167–3171
DOI: 10.1021/j100689a024
Well-cited paper detailing the catalytic effects of substituting Sr2+ for Ca2+ in hydroxyapatites for dehydration of alcohol —JPC Senior Editor Robert Dickson
***
(AN#39) – Y – Yttrium

Mass Spectroscopic and ESR Characterization of Soluble Yttrium-Containing Metallofullerenes YC82 And Y2C82
J. Phys. Chem., 1992, 96 (9), pp 3571–3573
DOI: 10.1021/j100188a004
Solvent soluble, yttrium-containing fullerenes are produced and characterized by mass spectroscopic analysis —JPC Senior Editor Stephan Link
***
(AN#40) – Zr – Zirconium

Adsorption of Ordered Zirconium Phosphonate Multilayer Films on Silicon and Gold Surfaces
J. Phys. Chem., 1988, 92 (9), pp 2597–2601
DOI: 10.1021/j100320a040
This was one of the first papers that showed multilayer zirconium phosphonate films can be prepared on silicon and gold substrates via sequential adsorption of their zirconium and phosphonic acid components —JPC Senior Editor Theodore Goodson, III
***
(AN#41) – Nb – Niobium

The Permeability of Niobium to Hydrogen
J. Phys. Chem., 1962, 66 (2), pp 351–353
DOI: 10.1021/j100808a038
The permeability of niobium metal to hydrogen gas was measured at temperature and pressure ranges of 950-1065 oC and 1.1-2.0 atm, respectively, and it was fit to a simple Arrhenius rate expression with an activation energy of 5.2 kcal/mol —JPC Senior Editor Timothy Minton
***
(AN#42) – Mo – Molybdenum
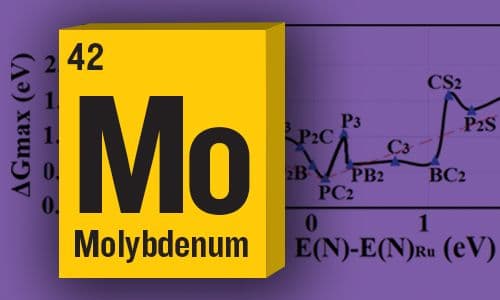
Computational Design of Single-Molybdenum Catalysts for the Nitrogen Reduction Reaction
J. Phys. Chem. C, 2019, 123 (4), pp 2347–2352
DOI: 10.1021/acs.jpcc.8b11509
Computational design of single-Molybdenum catalysts for nitrogen reduction offering overall overpotentials lower than 0.60 V —JPC Senior Editor Victor Batista
***
(AN#43) – Tc – Technetium

Report on the Occurrence of Technetium on the Earth’s Crust
J. Phys. Chem., 1956, 60 (6), pp 707–715
DOI: 10.1021/j150540a002
Hard to prove a negative, but this paper attempts to demonstrate the non-existence of terrestrial Tc through analysis of geologic samples most likely to contain the element —JPC Senior Editor William Schneider
***
(AN#44) – Ru – Ruthenium

Excited States of Mixed Ligand Chelates of Ruthenium(II) and Rhodium(III)
J. Phys. Chem., 1976, 80 (20), pp 2206–2211
DOI: 10.1021/j100561a016
It has the charming advantage that it was part of a “discussion” so the authors answer questions about it at the end of the paper! —JPC C Deputy Editor Catherine J. Murphy
***
(AN#45) – Rh – Rhodium

Infrared Studies of Carbon Monoxide Chemisorbed on Rhodium
J. Phys. Chem., 1957, 61 (11), pp 1504–1512
DOI: 10.1021/j150557a013
Infrared transmission spectrum of CO on Rh is described as involving three surface species —JPC Senior Editor Xueming Yang
***
(AN#46) – Pd – Palladium

Diffusion and Solubility of Hydrogen in Palladium and Palladium–Silver Alloys
J. Phys. Chem., 1970, 74 (3), pp 503–511
DOI: 10.1021/j100698a005
Pd is a key hydrogenation catalyst and this paper first measures the H diffusion and solubility in Pd and Pd-Ag —JPC Senior Editor Zhi-Pan Liu
***
(AN#47) – Ag – Silver

The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment
J. Phys. Chem. B, 2003, 107 (3), pp 668–677
DOI: 10.1021/jp026731y
Feature Article describing the progress made up to 2003 on the theory of nanoparticle optical properties, with emphasis on light scattering from particles of arbitrary shape in complex environments, and discussing spherical and triangular silver particles as an example of particular interest —JPC Senior Editor Francisco Zaera
***
(AN#48) – Cd – Cadmium

Two-Dimensional Electronic Spectroscopy Unravels sub-100 fs Electron and Hole Relaxation Dynamics in Cd-Chalcogenide Nanostructures
J. Phys. Chem. Lett., 2017, 8 (10), pp 2285–2290
DOI: 10.1021/acs.jpclett.7b00682
Two-Dimensional Electronic Spectroscopy Unravels sub-100 fs Electron and Hole Relaxation Dynamics in Cd-Chalcogenide Nanostructures —JPC Letters Deputy Editor Gregory D. Scholes
***
(AN#49) – In – Indium
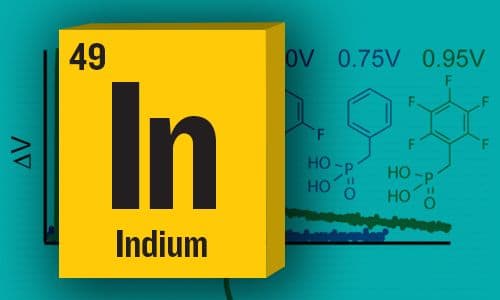
ITO Interface Modifiers Can Improve VOC in Polymer Solar Cells and Suppress Surface Recombination
J. Phys. Chem. Lett., 2013, 4 (23), pp 4038–4044
DOI: 10.1021/jz4021525
Linear dependence of the open-circuit voltage of organic photovoltaic devices on the modified indium tin oxide (ITO) work function —JPC Senior Editor Juan Bisquert
***
(AN#50) – Sn – Tin

Capped Semiconductor Colloids. Synthesis and Photoelectrochemical Behavior of TiO2 Capped SnO2 Nanocrystallites
J. Phys. Chem., 1995, 99 (22), pp 9182–9188
DOI: 10.1021/j100022a035
An investigation on the effect of TiO2 capping on SnO2 colloidal nanocrystallites for photoelectrochemical applications —JPC Senior Editor Jin Zhang
***
(AN#51) – Sb – Antimony

Stability of Free Intermetallic Compound Clusters: Lead/Antimony and Bismuth/Antimony
J. Phys. Chem., 1987, 91 (10), pp 2649–2653
DOI: 10.1021/j100294a037
In the gas phase antimony atoms form tetrahedra, and this is the starting point for the understanding of lead-antimony and bismuth-antimony clusters —JPC Senior Editor Neil Snider
***
(AN#52) – Te – Tellurium

Intrinsic Piezoelectricity in Two-Dimensional Materials
J. Phys. Chem. Lett., 2012, 3 (19), pp 2871–2876
DOI: 10.1021/jz3012436
Tellurium-piezoelectricity of two dimensional MoTe2 and WTe2 is predicted; highly cited paper —JPC Senior Editor Oleg Prezhdo
***
(AN#53) – I – Iodine

Photodissociation and Geminate Recombination Dynamics of Iodine (I2-) in Mass-Selected Iodine-Carbon Dioxide (I2-(CO2)n) Cluster Ions
J. Phys. Chem., 1991, 95 (21), pp 8028–8040
DOI: 10.1021/j100174a008
I2-(CO2)n cluster photodissociation, geminate recombination —JPC Senior Editor Timothy Zwier
***
(AN#54) – Xe – Xenon

Distribution and Dynamic Properties of Xenon Dissolved in the Ionic Smectic Phase of [C16mim][NO3]: MD Simulation and Theoretical Model
J. Phys. Chem. B, 2016, 120 (9), pp 2578–2585
DOI: 10.1021/acs.jpcb.5b12470
Macroscopic transport coefficients are calculated for Xenon in an ionic liquid, as it diffuses across the ionic layers of the smectic phase from a hydrophobic, alkyl environment —JPC Senior Editor Tanja Cuk
***
[/expand]
Period 6
(AN#55) – Cs – Cesium
Many-Body Effects Determine the Local Hydration Structure of Cs+ in Solution
J. Phys. Chem. Lett., 2019, 10 (3), pp 406–412
DOI: 10.1021/acs.jpclett.8b03829
MD simulations of Cs+ hydration demonstrates the importance of polarization effects —JPC Senior Editor Haizheng Zhong
***
(AN#56) – Ba – Barium

Structures of the Ferroelectric Phases of Barium Titanate
J. Phys. Chem., 1993, 97 (10), pp 2368–2377
DOI: 10.1021/j100112a043
Structure of ferroelectric phases of BaTiO3 determined by Rietveld refinement using powder diffraction data collected at a spallation neutron source —JPC Senior Editor Andrew Gewirth
***
(AN#57) – La – Lanthanum

Fullerenes with Metals Inside
J. Phys. Chem., 1991, 95 (20), pp 7564–7568
DOI: 10.1021/j100173a002
Letter reports the observation of fullerenes with single lanthanum atoms caged inside —JPC Senior Editor Amy Mullin
***
(AN#58) – Ce – Cerium
Direct Evidence for Hydroxyl Radical Scavenging Activity of Cerium Oxide Nanoparticles
J. Phys. Chem. C, 2011, 115 (11), pp 4433–4438
DOI: 10.1021/jp109819u
Studies the mechanism of antioxidant behavior of Cerium Oxide nano-particles —JPC Senior Editor Arun Yeithiraj
***
(AN#59) – Pr – Praseodymium

The Effect of LiClO4, LiCl, and LiBr on the Polarographic Behavior and Ultraviolet Spectrum of Praseodymium(III) in Ethanol
J. Phys. Chem., 1963, 67 (6), pp 1275–1278
DOI: 10.1021/j100800a027
Paper on effects of different anions on Pr(III) in solution —JPC Senior Editor Benjamin Schwartz
***
(AN#60) – Nd – Neodymium

Enhanced Emission of Deuterated Tris(hexafluoroacetylacetonato)neodymium(III) Complex in Solution by Suppression of Radiationless Transition via Vibrational Excitation
J. Phys. Chem., 1996, 100 (24), pp 10201–10205
DOI: 10.1021/jp960290z
A study of the impact of various deuterated solvents on a Nd3+ complex (150 citations) —JPC Senior Editor Daniel Crawford
***
(AN#61) – Pm – Promethium

Calorimetric Determination of the Mean β-Energy and Half-Life of Promethium-147
J. Phys. Chem., 1965, 69 (4), pp 1220–1223
DOI: 10.1021/j100888a021
The authors determine the mean β-particle energy and the half-life of promethium-147 —JPC Senior Editor Eric Weitz
***
(AN#62) – Sm – Samarium

The Near Infrared Transitions of the Trivalent Lanthanides in Solution. I. Praseodymium(III), Neodymium(III), Samarium(III), and Europium(III) Ions
J. Phys. Chem., 1962, 66 (11), pp 2159–2164
DOI: 10.1021/j100817a020
Report of NIR transitions of molten trivalent lanthanides —JPC Senior Editor Franz Geiger
***
(AN#63) – Eu – Europium

Hierarchical Emergence and Dynamic Control of Chirality in a Photoresponsive Dinuclear Complex
J. Phys. Chem. Lett., 2018, 9 (9), pp 2151–2157
DOI: 10.1021/acs.jpclett.8b00690
Hierarchical Emergence and Dynamic Control of Chirality in a Photoresponsive Dinuclear Complex —JPC Letters Deputy Editor Gregory D. Scholes
***
(AN#64) – Gd – Gadolinium

Water-Exchange, Electronic Relaxation, and Rotational Dynamics of the MRI Contrast Agent [Gd(DTPA-BMA)(H2O)] in Aqueous Solution: A Variable Pressure, Temperature, and Magnetic Field Oxygen-17 NMR Study
J. Phys. Chem., 1994, 98 (1), pp 53–59
DOI: 10.1021/j100052a010
17O NMR longitudinal and transverse relaxation rates and chemical shifts were measured for a Gadolinium complex as a measure of the efficacy as a magnetic resonance imaging contrast agent —JPC Senior Editor Gemma Solomon
***
(AN#65) – Tb – Terbium

Rare Earths. II. A Mass Spectrometric Determination of the Heats of Sublimation (or Vaporization) of Neodymium, Praseodymium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium and Lutetium
J. Phys. Chem., 1961, 65 (8), pp 1404–1409
DOI: 10.1021/j100826a030
Initial measurement of mass spectrometry determination of heat of sublimation of seven rare earth metals including Terbium, Holmium, Erbium and Lutetium —JPC Senior Editor Gang-yu Liu
***
(AN#66) – Dy – Dysprosium

Synthesis, Structure, Photoluminescence, and Electroluminescence Properties of a New Dysprosium Complex
J. Phys. Chem. C, 2007, 111 (5), pp 2295–2300
DOI: 10.1021/jp064749t
A new dysprosium complex was synthesized and its structural and photophysical properties were evaluated. The energy transfer mechanism between the ligand and central Dy3+ ion is discussed, and a series of electroluminescent devices were built. Emission at both yellow (572nm) and blue (480nm) wavelengths was observed. Appropriate choice of the yellow/blue intensity ratio is proposed to be tunable to achieve white luminescent emission. —JPC Senior Editor Gillian R. Goward
***
(AN#67) – Ho – Holmium

Amplifying Excitation-Power Sensitivity of Photon Upconversion in a NaYbF4:Ho Nanostructure for Direct Visualization of Electromagnetic Hotspots
J. Phys. Chem. Lett., 2016, 7 (23), pp 4916–4921
DOI: 10.1021/acs.jpclett.6b02210
Holmium ion doped nanoparticles produce upconverted luminescence that can be used for imaging hot-spots in plasmonic materials —JPC Senior Editor Gregory V. Hartland
***
(AN#68) – Er – Erbium

Highly Efficient Multicolor Up-Conversion Emissions and Their Mechanisms of Monodisperse NaYF4:Yb,Er Core and Core/Shell-Structured Nanocrystals
J. Phys. Chem. C, 2007, 111 (37), pp 13721–13729
DOI: 10.1021/jp073920d
Synthesis of highly efficient erbium doped phosphors and their mechanism of efficient up-conversion —JPC Senior Editor Howard Fairbrother
***
(AN#69) – Tm – Thulium

The Electronic and Vibrational Structure of Endohedral Tm3N@C80 (I) Fullerene − Proof of an Encaged Tm3+
J. Phys. Chem. A, 2005, 109 (32), pp 7088–7093
DOI: 10.1021/jp0525080
Characterization of Encaged Tm3+ in C80 fullerene —JPC Senior Editor Hua Guo
***
(AN#70) – Yb – Ytterbium

Visible Upconversion in Rare Earth Ion-Doped Gd2O3 Nanocrystals
J. Phys. Chem. B, 2004, 108 (50), pp 19205–19209
DOI: 10.1021/jp048072q
A landmark paper in the rare-earth upconversion literature —JPC Senior Editor John Fourkas
***
(AN#71) – Lu – Lutetium

Electrical and Magnetic Properties of Liquid Crystalline Molecular Materials: Lithium and Lutetium Phthalocyanine Derivatives
J. Phys. Chem., 1989, 93 (24), pp 8105–8110
DOI: 10.1021/j100361a026
Double decker lutetium phthalocyanine based liquid crystalline molecular materials with interesting properties and applications —JPC Senior Editor Kankan Bhattacharyya
***
(AN#72) – Hf – Hafnium

Atomic Layer Deposition of Hafnium Oxide from Tetrakis(ethylmethylamino)hafnium and Water Precursors
J. Phys. Chem. C, 2007, 111 (17), pp 6495–6499
DOI: 10.1021/jp070362u
Thermodynamics of hafnium calculated to predict atomic layer deposition processes —JPC Senior Editor Martin Zanni
***
(AN#73) – Ta – Tantalum

Quantum-Sized PbS, CdS, Ag2S, Sb2S3, and Bi2S3 Particles as Sensitizers for Various Nanoporous Wide-Bandgap Semiconductors
J. Phys. Chem., 1994, 98 (12), pp 3183–3188
DOI: 10.1021/j100063a022
Paper investigates the sensitization of nanoporous oxides (including tantalum oxide) by quantum-sized metal sulfides —JPC Senior Editor Benedetta Mennucci
***
(AN#74) – W – Tungsten

Photoelectrochemistry of Quantized Tungsten Trioxide Colloids: Electron Storage, Electrochromic, and Photoelectrochromic Effects
J. Phys. Chem., 1993, 97 (42), pp 11064–11070
DOI: 10.1021/j100144a027
Tungsten oxide (WO3) colloidal particles have been shown to act as electron-relay systems with potential for construction of solar energy conversion and storage devices —JPC Senior Editor Pavel Jungwirth
***
(AN#75) – Re – Rhenium

Surface Catalytic Sites Prepared from [HRe(CO)5] and [H3Re3(CO)12]: Mononuclear, Trinuclear, and Metallic Rhenium Catalysts Supported on Magnesia
J. Phys. Chem., 1990, 94 (22), pp 8439–8450
DOI: 10.1021/j100385a017
Spectroscopic and structural characterization of various heterogeneous Re catalysts on MgO, providing a better understanding of catalytic activity —JPC Senior Editor Robert Dickson
***
(AN#76) – Os – Osmium

Application of the Energy Gap Law to Excited-State Decay of Osmium(II)-Polypyridine Complexes: Calculation of Relative Nonradiative Decay Rates from Emission Spectral Profiles
J. Phys. Chem., 1986, 90 (16), pp 3722–3734
DOI: 10.1021/j100407a046
Radiative and nonradiative decay rates of the metal-to-ligand charge-transfer excited states for osmium complexes scale in accordance with the Einstein law for spontaneous emission and the energy gap law, respectively —JPC Senior Editor Stephan Link
***
(AN#77) – Ir – Iridium

A High Yield Synthesis of Ligand-Free Iridium Oxide Nanoparticles with High Electrocatalytic Activity
J. Phys. Chem. Lett., 2011, 2 (5), pp 402–406
DOI: 10.1021/jz200051c
Synthesis and characterization of IrOx nanoparticles, which are highly active for water oxidation —JPC Editor-in-Chief George C. Schatz
***
(AN#78) – Pt – Platinum

Platinum Black Catalysts
J. Phys. Chem., 1930, 34 (4), pp 748–752
DOI: 10.1021/j150310a007
Platinum black was prepared by three methods, and its catalytic activity to several oxidation and reduction reactions was correlated with particle size, with the smaller particle size showing higher catalytic activity —JPC Senior Editor Timothy Minton
***
(AN#79) – Au – Gold

Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols
J. Phys. Chem., 1982, 86 (17), pp 3391–3395
DOI: 10.1021/j100214a025
Classic paper on colloidal gold (and silver) —JPC C Deputy Editor Catherine J. Murphy
***
(AN#80) – Hg – Mercury

Mercury Drop “Attacks” an Oxidant Crystal
J. Phys. Chem. B, 2000, 104 (15), pp 3589–3593
DOI: 10.1021/jp9936502
A mercury drop “attacks” a K2Cr2O7 crystal placed ∼10 mm apart. The circular Hg drop began to elongate and to move toward the crystal with a shape and motion related to the anisotropy of the interfacial tension —JPC Senior Editor Victor Batista
***
(AN#81) – Tl – Thallium

Viscosity and Self-Diffusion of Liquid Thallium from Its Melting Point to About 1300°K
J. Phys. Chem., 1965, 69 (2), pp 518–521
DOI: 10.1021/j100886a026
Great example of how physical property measurements were at one time an important part of the journal, here in the context of a less common element —JPC Senior Editor William Schneider
***
(AN#82) Pb – Lead

Interband and Intraband Optical Studies of PbSe Colloidal Quantum Dots
J. Phys. Chem. B, 2002, 106 (41), pp 10634–10640
DOI: 10.1021/jp021187e
Interband and intraband optical studies of PbSe colloidal quantum dots —JPC Senior Editor Xueming Yang
***
(AN#83) – Bi – Bismuth

The Bismuth-Bismuth Tribromide and Bismuth-Bismuth Triiodide Systems
J. Phys. Chem., 1962, 66 (1), pp 28–31
DOI: 10.1021/j100807a006
Paper concerned with industrial applications —JPC C Deputy Editor Catherine J. Murphy
***
(AN#84) – Po – Polonium

The Preparation and Identification of Some Intermetallic Compounds of Polonium
J. Phys. Chem., 1960, 64 (4), pp 434–440
DOI: 10.1021/j100833a014
The finding of Po-forming solid alloy with other elements —JPC Senior Editor Zhi-Pan Liu
***
(AN#85) – At – Astatine
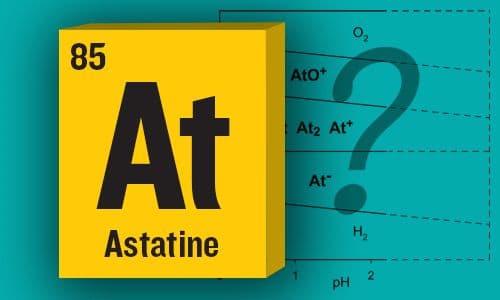
Astatine Standard Redox Potentials and Speciation in Acidic Medium
J. Phys. Chem. A, 2010, 114 (1), pp 576–582
DOI: 10.1021/jp9077008
A combined experimental and theoretical study that shows that At can be present in aqueous solution in three forms, namely, At−, At+, and AtO+, in the 1−2 pH range, and that the oxidation state 0 cannot exist —JPC Senior Editor Francisco Zaera
***
(AN#86) – Rn – Radon

Adsorption of Radon on Metal Surfaces: A Model Study for Chemical Investigations of Elements 112 and 114
J. Phys. Chem. B, 2002, 106 (21), pp 5413–5420
DOI: 10.1021/jp015553q
There are more highly-cited papers, but they usually include radon with a bunch of other noble gases —JPC C Deputy Editor Catherine J. Murphy
***
[/expand]
Period 7
(AN#87) – Fr – Francium
First Experimentally Determined Thermodynamic Values of Francium: Hydration Energy, Energy of Partitioning, and Thermodynamic Radius
J. Phys. Chem. B, 2013, 117 (31), pp 9258–9261
DOI: 10.1021/jp401880f
Experimental measurement of Fr+ ionic radius resulted in smaller radius than previously
thought —JPC Editor-in-Chief George C. Schatz
***
(AN#88) – Ra – Radium
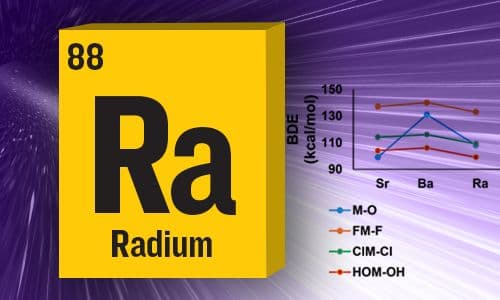
Structures and Heats of Formation of Simple Alkaline Earth Metal Compounds II: Fluorides, Chlorides, Oxides, and Hydroxides for Ba, Sr, and Ra
J. Phys. Chem. A, 2018, 122 (1), pp 316–327
DOI: 10.1021/acs.jpca.7b09056
Relativistic Coupled Cluster theory has been used to calculate Heats of Formation
of compounds of Ra —JPC Senior Editor Kankan Bhattacharyya
***
(AN#89) – Ac – Actinium

Capped Semiconductor Colloids. Synthesis and Photoelectrochemical Behavior of TiO2-Capped SnO2 Nanocrystallites
J. Phys. Chem., 1995, 99 (22), pp 9182–9188
DOI: 10.1021/j100022a035
The volatility of actinium was measured through volatilization experiments at high temperature —JPC Senior Editor Jin Zhang
***
(AN#90) – Th – Thorium

Making and Breaking Bonds in the Solid State: The Thorium Chromium Silicide (ThCr2Si2) Structure
J. Phys. Chem., 1985, 89 (20), pp 4175–4181
DOI: 10.1021/j100266a007
Highly cited feature article by Roald Hoffman on crystal structures for which ThCr2Si2 is a prototype —JPC Senior Editor John Fourkas
***
(AN#91) – Pa – Protactinium

Theoretical Study on Molecular Property of Protactinium(V) and Uranium(VI) Oxocations: Why Does Protactinium(V) Form Monooxo Cations in Aqueous Solution?
J. Phys. Chem. A, 2006, 110 (49), pp 13303–13309
DOI: 10.1021/jp0641435
Electronic structure calculation of Pa(V) oxocation in aqueous solution clearly support from an energetic point of view the experimental result that monooxo protactinyl cation, PaO3+, is a preferable species for Pa(V)aq —JPC Senior Editor Pavel Jungwirth
***
(AN#92) – U – Uranium

The Uranium–Oxygen System: UO2.5 to U3O8
J. Phys. Chem., 1955, 59 (2), pp 136–138
DOI: 10.1021/j150524a010
Uranium-Oxygen system: UO2.5 to U3O8 —JPC Senior Editor Timothy Zwier
***
(AN#93) – Np – Neptunium

Measurement of the Absorption Spectra of Neptunium Ions in Heavy Water Solution from 0.35 to 1.85 μ
J. Phys. Chem., 1958, 62 (3), pp 382–383
DOI: 10.1021/j150561a050
Examines the adsorption spectrum of neptunium in heavy water —JPC Senior Editor Andrew Gewirth
***
(AN#94) – Pu – Plutonium

The Kinetics of the Reaction Between Plutonium(VI) and Iron(II)
J. Phys. Chem., 1963, 67 (7), pp 1425–1432
DOI: 10.1021/j100801a005
Classic 1962 study of rates and thermodynamics of the oxidation-reduction reaction of plutonium and iron —JPC Senior Editor Amy Mullin
***
(AN#95) – Am – Americium

Americium Organometallic Ions Produced by Laser Ablation of AmO2 in Polyimide
J. Phys. Chem. A, 1998, 102 (24), pp 4501–4508
DOI: 10.1021/jp981145j
Americium organometallic ions produced by laser ablation of AmO2 in polyimide —JPC Senior Editor Arun Yeithiraj
***
(AN#96) – Cm – Curium

Hydration Shell Structure and Dynamics of Curium(III) in Aqueous Solution: First Principles and Empirical Studies
J. Phys. Chem. A, 2011, 115 (18), pp 4665–4677
DOI: 10.1021/jp201043f
Simulation paper on the structure of Cm(III) in aqueous solution —JPC Senior Editor Benjamin Schwartz
***
(AN#97) – Bk – Berkelium

Electron-Transfer Spectra and the II-III Oxidation Potentials of Some Lanthanide and Actinide Halides in Solution
J. Phys. Chem., 1969, 73 (4), pp 1177–1178
DOI: 10.1021/j100724a084
Includes calculated absorption maximum and oxidation potential of BkBr —JPC Senior Editor Daniel Crawford
***
(AN#98) – Cf – Californium

Radiolysis of 0.4 M Sulfuric Acid Solutions with Fission Fragments from Dissolved Californium-252. Estimated Yields of Radical and Molecular Products that Escape Reactions in Fission Fragment Tracks
J. Phys. Chem., 1975, 79 (19), pp 1991–1995
DOI: 10.1021/j100586a002
A study of the radiolysis of sulfuric acid solution, with and without Fe2+ and Ce4+ ions, using dissolved californium-252 —JPC Senior Editor Eric Weitz
***
(AN#99) – Es – Einsteinium

Intramolecular Energy Transfer and Sensitized Luminescence in Actinide(III) β-Diketone Chelates
J. Phys. Chem., 1969, 73 (5), pp 1540–1549
DOI: 10.1021/j100725a060
First measurements of Es(III) chelate lifetimes from the transuranium laboratory at ORNL —JPC Senior Editor Franz Geiger
***
(AN#100) – Fm – Fermium

Electron-Transfer Spectra and the II-III Oxidation Potentials of Some Lanthanide and Actinide Halides in Solution
J. Phys. Chem., 1969, 73 (4), pp 1177–1178
DOI: 10.1021/j100724a084
Calculated oxidation potential (II-III) and lowest charge-transfer band for a Fermium bromide —JPC Senior Editor Gemma Solomon
***
(AN#101) – Md – Mendelevium

Role of Atomic Electronics in f-Element Bond Formation: Bond Energies of Lanthanide and Actinide Oxide Molecules
J. Phys. Chem. A, 2003, 107 (39), pp 7891–7899
DOI: 10.1021/jp035003n
This paper makes estimates of the bond dissociation enthalpies of all the actinide monoxides —JPC Editor-in-Chief George C. Schatz
***
(AN#102) – No – Nobelium

Role of Atomic Electronics in f-Element Bond Formation: Bond Energies of Lanthanide and Actinide Oxide Molecules
J. Phys. Chem. A, 2003, 107 (39), pp 7891–7899
DOI: 10.1021/jp035003n
The bond dissociation energy of NoO cannot be estimated with confidence —JPC Editor-in-Chief George C. Schatz
***
(AN#103) – Lr – Lawrencium

Redox Reactions for Group 5 Elements, Including Element 105, in Aqueous Solutions
J. Phys. Chem., 1992, 96 (26), pp 11096–11101
DOI: 10.1021/j100205a086
Calculation of ionization potentials using the multiconfiguration Dirac-Fock method, that shows that the maximum oxidation state of Lr is more stable than the higher mass group 5 elements —JPC Senior Editor Gregory V. Hartland
***
(AN#104) – Rf – Rutherfordium

Total Energy Calculations of RfCl4 and Homologues in the Framework of Relativistic Density Functional Theory
J. Phys. Chem. A, 2000, 104 (27), pp 6495–6498
DOI: 10.1021/jp993980m
Demonstration that a four-component, fully relativistic density functional method could provide accurate values for the formation and binding energies of RfCl4 —JPC Senior Editor Howard Fairbrother
***
(AN#105) – Db – Dubnium

Thermochemical Characterization of Seaborgium Compounds in Gas Adsorption Chromatography
J. Phys. Chem. A, 1999, 103 (46), pp 9296–9306
DOI: 10.1021/jp9917751
Dubnium is mentioned in this paper, but no experimental data reported —JPC Senior Editor Hua Guo
***
(AN#106) – Sg – Seaborgium

Ionization Potentials of Seaborgium
J. Phys. Chem. A, 1999, 103 (42), pp 8458–8462
DOI: 10.1021/jp9903211
This is the first paper to attempt to calculate the ionization potentials of Seaborgium, which was officially only 2 years old at the time —JPC Senior Editor John Fourkas
***
(AN#107) – Bh – Bohrium

On the Stability and Volatility of Group 8 Tetroxides, MO4 (M = Ruthenium, Osmium, and Hassium (Z = 108))
J. Phys. Chem. B, 2002, 106 (26), pp 6679–6684
DOI: 10.1021/jp0257146
Bohrium is briefly mentioned in a study that mostly refers to Hs —JPC Senior Editor Kankan Bhattacharyya
***
(AN#108) – Hs – Hassium

On the Stability and Volatility of Group 8 Tetroxides, MO4 (M = Ruthenium, Osmium, and Hassium (Z = 108))
J. Phys. Chem. B, 2002, 106 (26), pp 6679–6684
DOI: 10.1021/jp0257146
The adsorption behavior of HsO4 on quartz surfaces is predicted based on its chemical stability —JPC Senior Editor Martin Zanni
***
(AN#109) – Mt – Meitnerium

N/A
***
(AN#110) – DS – Darmstadtium

N/A
***
(AN#111) – Rg – Roentgenium

Predicted Properties of the Superheavy Elements. II. Element 111, Eka-Gold
J. Phys. Chem., 1973, 77 (14), pp 1806–1809
DOI: 10.1021/j100633a017
Early (1973) electronic structure study comparing Rg and Au —JPC Senior Editor Theodore Goodson, III
***
(AN#112) – Cn – Copernicium

Atomic and Molecular Properties of Elements 112, 114, and 118
J. Phys. Chem. A, 2005, 109 (15), pp 3493–3500
DOI: 10.1021/jp050736o
Theoretical calculations of atoms and diatomic molecules of Elements 112, 114, and 118 were conducted, and the effects of spin-orbit coupling on the expected chemistry were examined. Element 112 (Copernicium) is similar in most respects to Hg, but the complex valence shell structure makes it intermediate in behavior between 6d and 7p transactinides —JPC Senior Editor Timothy Minton
***
(AN#113) – Nh – Nihonium

Atomic Properties of Element 113 and Its Adsorption on Inert Surfaces from ab Initio Dirac−Coulomb Calculations
J. Phys. Chem. A, 2008, 112 (51), pp 13712–13716
DOI: 10.1021/jp8061306
Dirac-Coulomb calculations with comparison with Tl —JPC Editor-in-Chief George C. Schatz
***
(AN#114) – Fl – Flerovium

Taming the Electronic Structure of Lead and Eka-lead (Flerovium) by the Relativistic Coupled Cluster Method
J. Phys. Chem. A, 2013, 117 (36), pp 8555–8567
DOI: 10.1021/jp402376b
For the very heaviest elements, trends in atomic properties (here ionization potential and excitation energies) are most readily probed with first principles calculations —JPC Senior Editor William Schneider
***
(AN#115) – Mc – Moscovium

Predicted Properties of the Superheavy Elements. III. Element 115, Eka-Bismuth
J. Phys. Chem., 1974, 78 (19), pp 1945–1949
DOI: 10.1021/j100612a015
Computational study comparing Mc with Bi —JPC Senior Editor Xueming Yang
***
(AN#116) – Lv – Livermorium
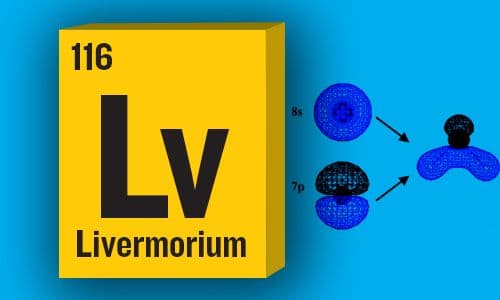
An Anomalous Bond Angle in (116)H2. Theoretical Evidence for Supervalent Hybridization
J. Phys. Chem. A, 2006, 110 (14), pp 4619–4621
DOI: 10.1021/jp060888z
Theoretical calculation shows the interesting structure of LvH2 structure due to spin-orbit coupling —JPC Senior Editor Zhi-Pan Liu
***
(AN#117) – Ts – Tennessine

Spin−Orbit Effects on the Electronic Structure of Heavy and Superheavy Hydrogen Halides: Prediction of an Anomalously Strong Bond in H[117]
J. Phys. Chem. A, 1999, 103 (5), pp 632–636
DOI: 10.1021/jp9843407
MRCI and CCSD(T) calculations of the bond energy and bond length of HTs show that the 8s orbital contributes significantly to bonding due to spin-orbit effects —JPC Editor-in-Chief George C. Schatz
***
(AN#118) – Og – Oganesson

Atomic and Molecular Properties of Elements 112, 114, and 118
J. Phys. Chem. A, 2005, 109 (15), pp 3493–3500
DOI: 10.1021/jp050736o
CCSD(T) study of Og suggests that it is much more reactive than Rn, with a Og2 bond energy of 0.062 eV, compared to 0.016eV for Rn2 —JPC Editor-in-Chief George C. Schatz
***
[/expand]
