The inherent chemistry of per- and polyfluoroalkyl substances (PFAS) contributes to their persistence in the environment. With increasing concerns about bioaccumulation, several new studies reveal promising potential for permanently destroying PFAS from both soil and water.
PFAS have been in use for decades across a huge range of industries, from paints to cookware. A key characteristic of PFAS is one of the strongest carbon–fluorine bonds ever, making them highly resistant to heat, stains, and water—and unfortunately, breakdown in the environment, earning them the nickname “forever chemicals”.1
Data suggest most people in the United States have been exposed to and carry PFAS in their blood, especially perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA).2 The Environmental Protection Agency lists several known health effects from PFAS, including decreased fertility, developmental effects, cancers, immune or hormonal disorders, and increased obesity risks.3
In response to public health fears, the World Health Organization is working on developing guidelines for PFAS in drinking water. Key principles outlined in the draft focus on preventing contamination, with the recommendation that non-essential uses of PFAS should be stopped.4 But alongside these efforts to reduce future use, a key question for chemists is not only how to remove PFAS pollutants from the environment, but also how to destroy them—from soil as well as water.
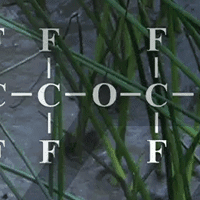
PFAS: A Look at Alternatives and Environmental Pollution
Recent work published in Environmental Science & Technology Letters describes a groundbreaking new method using ball-milling with boron nitride to destroy PFAS in sediment.5 Ball-milling is an alternative energy input method that has previously been used in organic reactions.6 The new piezoelectric-assisted process was built on the principle that collisions during milling can generate potentials for PFAS destruction without the need for solvents. This works because atomic displacement resulting from mechanical impact causes a mismatch between the cation and anion centers, generating a dipole moment.
In water, granular activated carbon (GAC) adsorption is currently the most widely used technology for treatment of PFAS. However, this process has its limitations, as GAC materials have a limited uptake capacity and are prone to PFAS breakthrough once a certain volume of water is treated. Additionally, the process generates PFAS-laden byproducts that require further treatment or disposal. One promising alternative solution for PFAS destruction in liquid is hydrothermal alkaline treatment (HALT).7,8 To achieve this, a strong alkali is added to water or soil slurry before it is subjected to high temperature and pressure, which degrades and defluorinates PFAS.7,8 Interestingly, HALT may also be a solution for treating PFAS-contaminated GAC.
Another possibility for PFAS destruction is direct oxidation in an electrochemical system. This type of elimination system usually focuses on direct anodic oxidation, while the cathodic reactions are generally overlooked. However, a recent study from a team at Tsinghua University, China, demonstrates that cathode-produced bubbles can result in energy-efficient removal of Perfluorooctanoic acid (PFOA).9
When compared to simple separation technologies, these novel approaches to destroy PFAS seem highly promising for addressing the long-term risks posed by these substances in the environment, and may one day soon be able to permanently reverse the status of PFAS as “forever chemicals.”
References
- Hogue, C. et al. A guide to the PFAS found in our environment. Chemical & Engineering News.
- Agency for Toxic Substances and Disease Registry, CDC. PFAS in the U.S. Population.
- United States Environmental Protection Agency. Our Current Understanding of the Human Health and Environmental Risks of PFAS.
- World Health Organization. PFOS and PFOA in Drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality.
- Yang, N. et al. Solvent-Free Nonthermal Destruction of PFAS Chemicals and PFAS in Sediment by Piezoelectric Ball Milling. Environ. Sci. Technol. Lett. 2023, 10, 2, 198–203.
- Xu, Q. et al. Ball-Milling: A Productive, Economical, and Widely Applicable Method for Condensation of Biomass-Derived Aldehydes and Ketones at Mild Temperatures. ACS Sustainable Chem. Eng. 2021, 9, 24, 8232–8237.
- Soke, O. et al. Application of Hydrothermal Alkaline Treatment to Spent Granular Activated Carbon: Destruction of Adsorbed PFASs and Adsorbent Regeneration. Environ. Sci. Technol. Lett. 2023, 10, 5, 425–430.
- Hao, S. et al. Application of Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Contaminated Groundwater and Soil. Environ. Sci. Technol. 2022, 56, 10, 6647–6657.
- Wan, Z. et al. Enhanced Electrochemical Destruction of Perfluorooctanoic Acid (PFOA) Aided by Overlooked Cathodically Produced Bubbles. Environ. Sci. Technol. Lett. 2023, 10, 1, 111–116.

