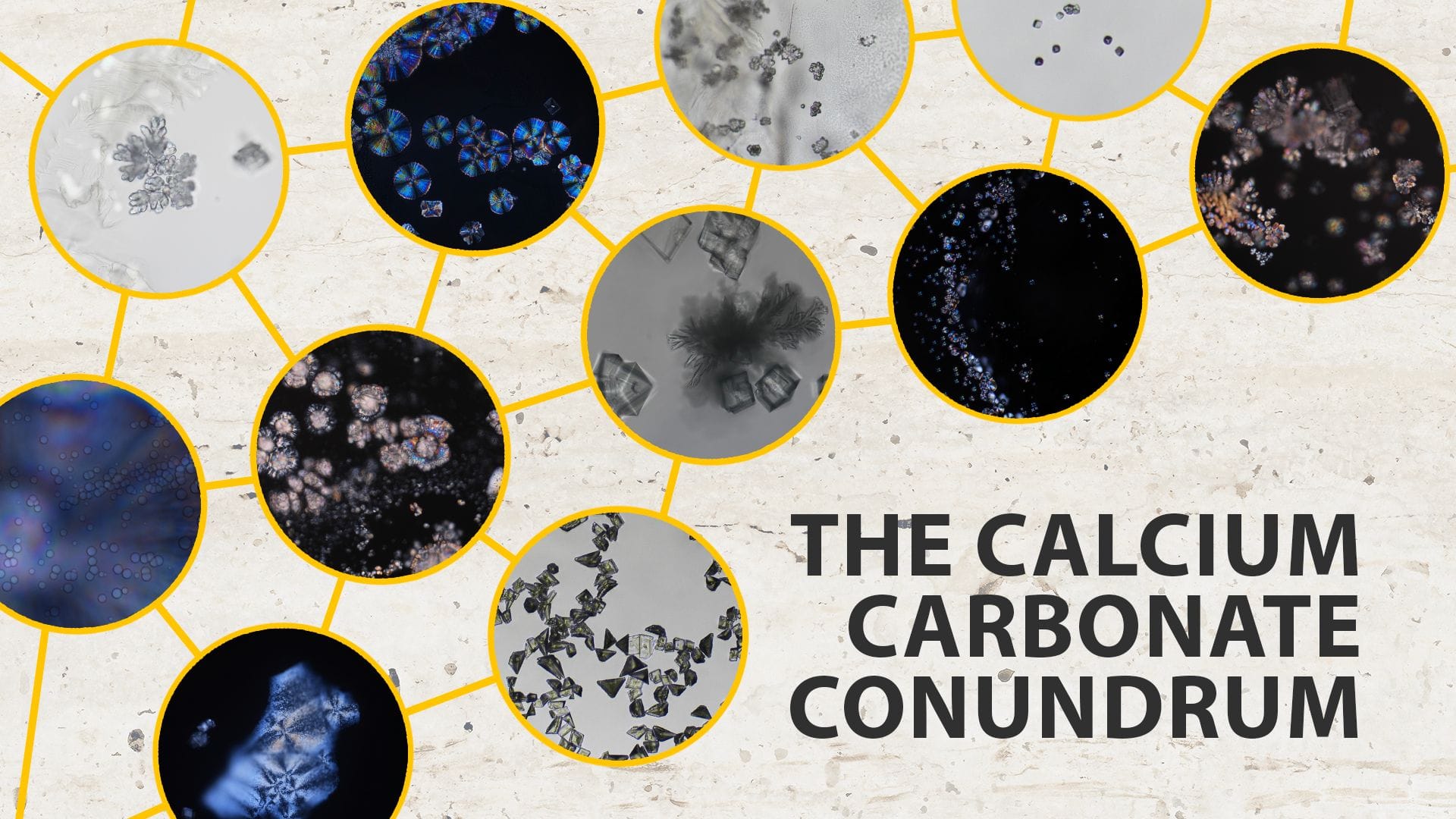
Calcium carbonate has been extensively studied across disciplines, but its crystallization process has been highly debated. Now, scientists have captured the beautiful, intricate formation of calcium carbonate crystals in real time.
Calcium carbonate (CaCO₃) is an insoluble white solid, widely used over many centuries for a number of purposes and studied across chemistry, biology, and geology.1 One of the most abundant materials on Earth, it is commonly recognized in its mineral form calcite, the chief component of limestone, marble, and chalk. Calcium carbonate also plays important roles in soil and marine ecosystems—many organisms, such as corals, mollusks, and some plankton, rely on it to build their shells and skeletons.
While at first glance it seems like a fairly simple molecule,2 calcium carbonate can create great complexity, forming hydrated crystallization structures through various mechanisms influenced by environmental conditions and impurities. In the 1880s, George Rainey first described the preparation and crystallization of amorphous calcium carbonate, but this is now something of a historical curiosity.3 Since then, its crystallization process has been highly debated, with contradicting claims made—possibly due to differences in initial conditions, precipitation protocols, and methods used. This scientific disagreement leaves an important gap in our knowledge, and understanding more about this process would be useful across many fields.
In a recent study published in Crystal Growth & Design, a team in Switzerland set out to gain more clarity on how calcium carbonate precipitates and crystallizes under varying conditions.4 Their approach combined optical microscopy with in situ Raman microspectroscopy, allowing them to directly observe and characterize the formation of calcium carbonate polymorphs as they appeared in real time. They found a surprisingly diverse and dynamic crystallization process—and we now have a front-row seat to these beautiful formations in the latest Headline Science video:
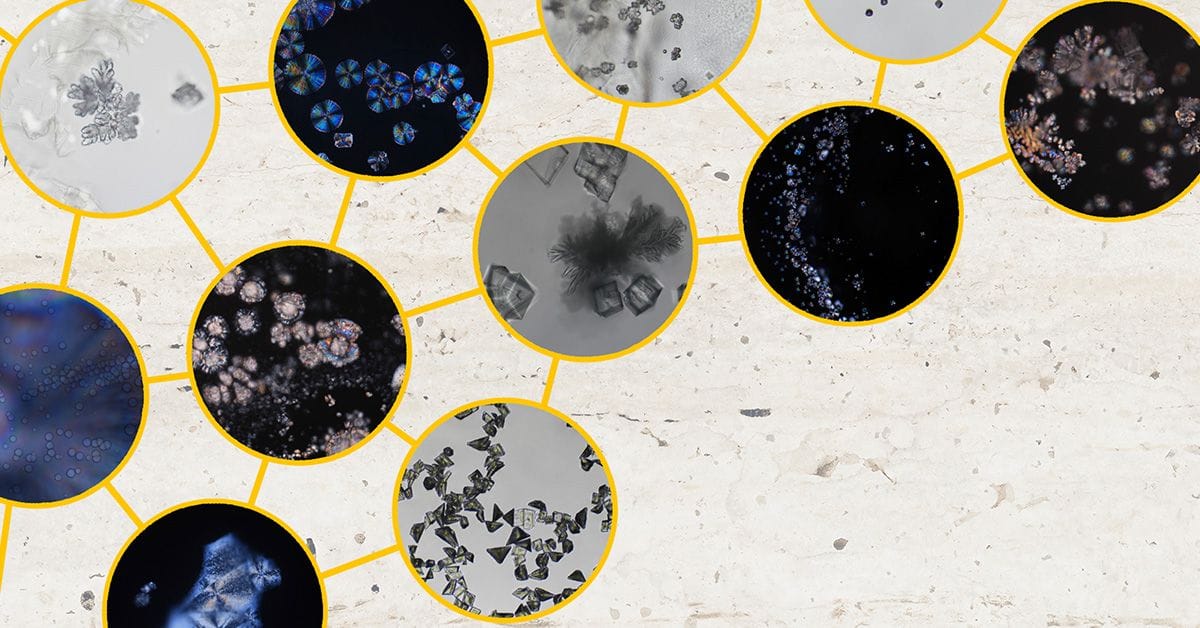
By varying the concentrations of calcium chloride and sodium carbonate solutions and adjusting the pH—without stirring or additives—the team identified five main stages in the crystallization process: gel, gel + amorphous calcium carbonate (ACC), pure ACC, crystalline phases, and metastable mixtures. Optical microscopy revealed a range of naturally forming morphologies previously reported only in the presence of additives.
"This study emphasizes the heterogeneity and complexity of calcium carbonate precipitation as well as the importance of initial conditions," note the authors in their article. "These optical images presented herein also remind us of how beautiful this system is and demonstrate the importance of studying the precipitation behaviors at the mesoscopic length scale."
Watch more Headline Science on YouTube!
The video above is brought to you by the ACS Science Communications team. To watch more exciting videos and shorts covering some of the latest research in ACS journals, visit the Headline Science page on YouTube.
Video credits:
Written and produced by Vangie Koonce
Editing and animations by Darren Weaver
Narrated by Jefferson Beck
Series produced by Vangie Koonce, Anne Hylden, Andrew Sobey, and Jefferson Beck
Executive produced by Matthew Radcliff
Explore related content from ACS Publications
Blog Post

Staying Cool with New Chalk-Coated Fabrics
ACS Journal Articles
Assembly of the Intraskeletal Coral Organic Matrix during Calcium Carbonate Formation
Silvia Milita*, Tal Zaquin, Simona Fermani, Devis Montroni, Iddo Pinkas, Luisa Barba, Giuseppe Falini*, and Tali Mass*
DOI: 10.1021/acs.cgd.3c00401
Nanocluster-Induced Liquid-like Precursor Formation and Crystallization: In Situ Visualization and 3D Reconstruction
Jin Chen, Guanbin Gao*, Zijun Zhang, Taolei Sun, Zhengyi Fu*, and Zhaoyong Zou*
DOI: https://pubs.acs.org/doi/10.1021/jacs.4c17643
Nineteenth Century Amorphous Calcium Carbonate
Bart Kahr*, Sophia Sburlati, Jackson Comes, John Mergo, Willem L. Noorduin, and Jong Seto*
DOI: 10.1021/acs.cgd.4c01066
Stearate-Coated Biogenic Calcium Carbonate from Waste Seashells: A Sustainable Plastic Filler
Maria Luisa Basile, Carla Triunfo, Stefanie Gärtner, Simona Fermani, Davide Laurenzi, Gabriele Maoloni, Martina Mazzon, Claudio Marzadori, Alessio Adamiano, Michele Iafisco, Devis Montroni, Jaime Gómez Morales, Helmut Cölfen, and Giuseppe Falini*
DOI: 10.1021/acsomega.3c06186
Control of Calcium Carbonate Crystallization in an Orthogonal Diffusion System
Yu Seob Shin, Gábor Holló, István Lagzi*, and Sung Ho Yang*
DOI: 10.1021/acs.cgd.4c01159
Amorphous Magnesium–Calcium Carbonate Phosphate: Crystallization Paced by the Reaction Solution Concentration
Yibo Zhang, Jia-hua Liu, Yun-chen Long, Xinxue Tang, Jing Zhong, Hanzhu Zhang, Jian Lu*, and Yang Yang Li*
DOI: 10.1021/acs.cgd.3c01420
References
- Calcium Carbonate. ACS Reagent Chemicals: Specifications and Procedures for Reagents and Standard-Grade Reference Materials, online ed., Part 4; American Chemical Society, 2017. DOI: 10.1021/acsreagents
- Calcite. Molecule of the Week Archive, American Chemical Society, 2005. https://www.acs.org/molecule-of-the-week/archive/c/calcite.html.
- Kahr, B. et al. Nineteenth Century Amorphous Calcium Carbonate. Cryst. Growth Des. 2024, 24, 22, 9301–9312.
- Barbosa, N. et al. Beauty and Complexity of Calcium Carbonate Precipitation: Optical Microscopy and in situ Raman Microspectroscopy Characterization. Cryst. Growth Des. 2025, 25, 2, 210–224.